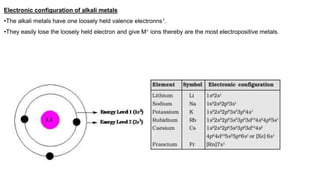
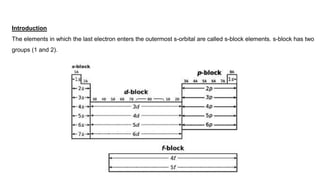
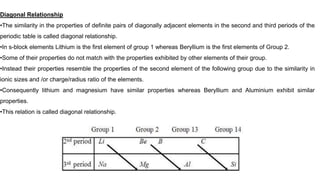
Dr. Sonia Rani presents information on s-block elements and their properties. S-block elements have electrons that enter the outermost s-orbital and include groups 1 and 2 of the periodic table. Group 1 elements are alkali metals that form strongly alkaline hydroxides when reacting with water. Group 2 elements are alkaline earth metals whose oxides and hydroxides are also alkaline. Diagonal relationships exist between lithium/magnesium and beryllium/aluminum due to their similar ionic sizes and charge/radius ratios. Washing soda, or sodium carbonate, is used for softening water and cleaning and exists as a decahydrate that loses water of crystallization when heated



![Similarity of beryllium and aluminium
•The charge/radius ratio of Be2+ ion is nearly the same as that of the Al3+ ion and hence exhibit similar properties.
•Both remains unaffected by acids due to the presence of an oxide film on the metallic surface.
•Hydroxide of both the elements dissolves in excess of alkali to produce beryllate ion[Be (OH)4]2– and aluminate ion
[Al(OH)4]–
•Chlorides of both the elements are soluble in organic solvents and act as strong Lewis acids
•The ions of both the elements have strong tendency to form complexes like BeF4
2–, AlF6
3–.](https://image.slidesharecdn.com/sblockelement-230915163552-bd965c40/85/s-block-element-pptx-6-320.jpg)