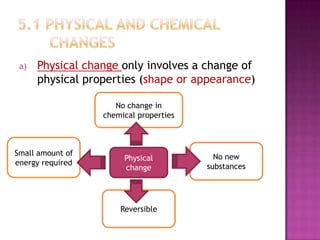
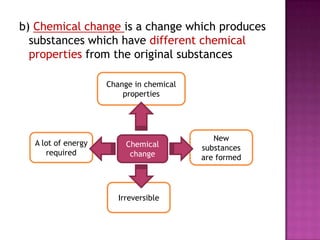
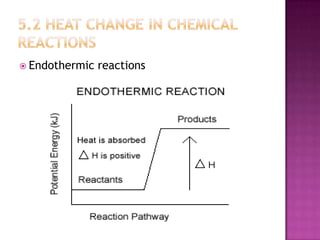
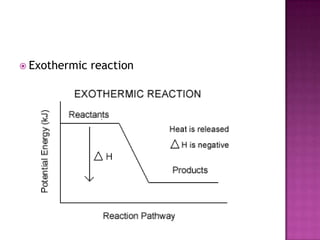
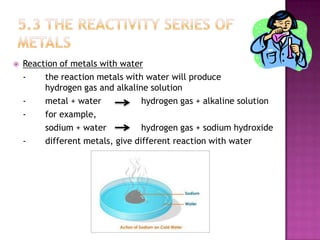
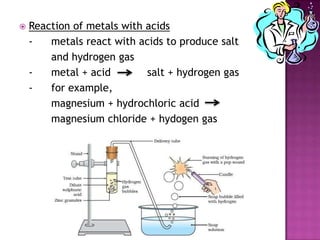
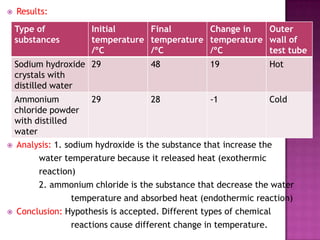
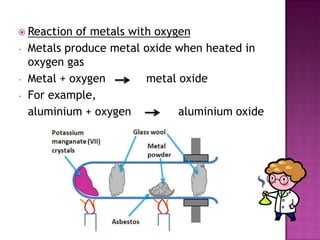
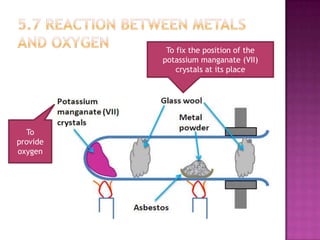
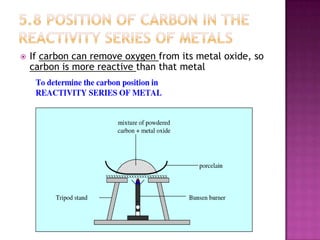
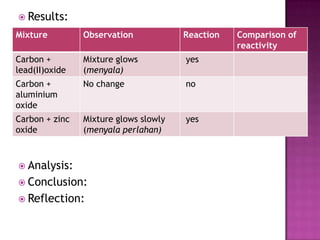
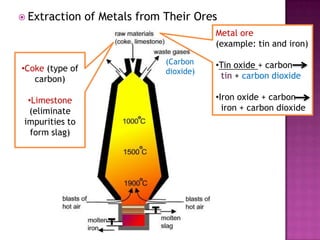
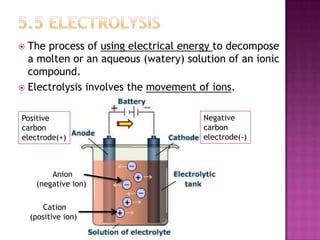
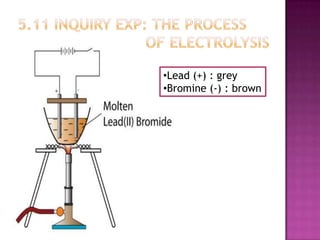
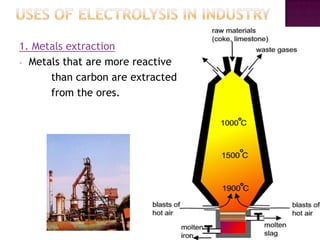
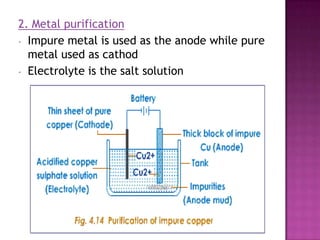
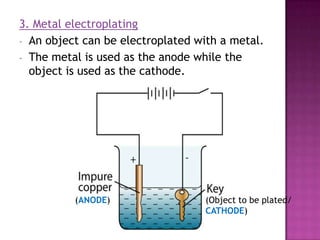
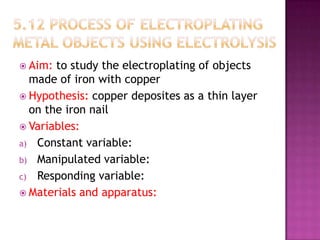
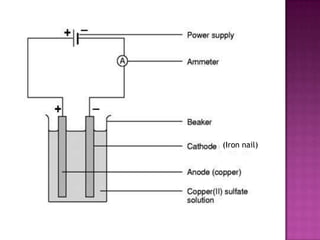
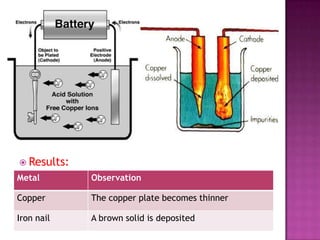
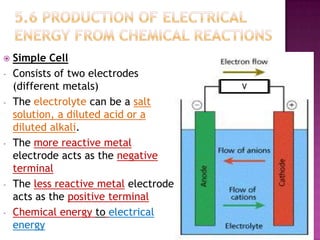
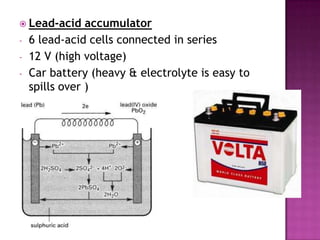
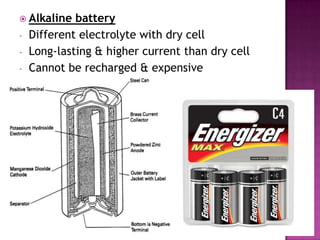
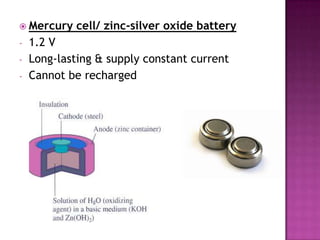
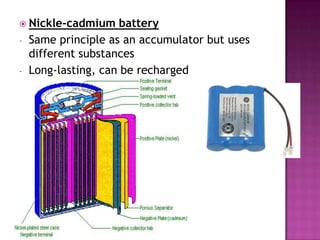
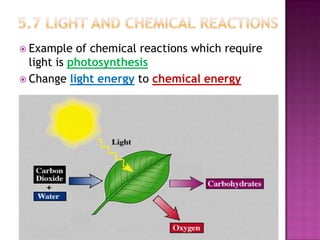
This document discusses different types of chemical and physical changes. It explains that physical changes only alter the physical properties of a substance and are reversible, while chemical changes produce new substances through irreversible reactions and often require more energy. Examples of each type of change are provided. The document also covers endothermic and exothermic reactions, noting that endothermic reactions absorb heat while exothermic reactions release heat.