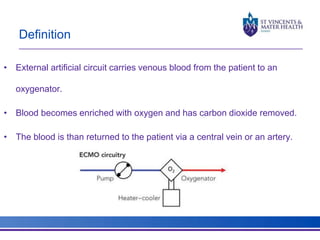
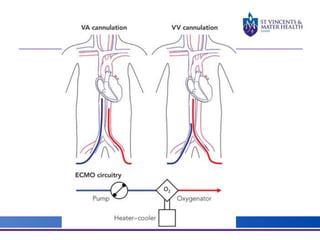
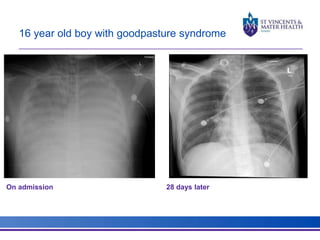
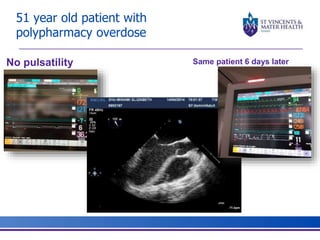
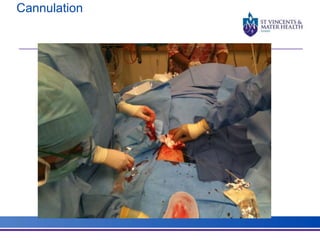
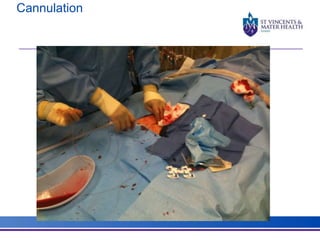
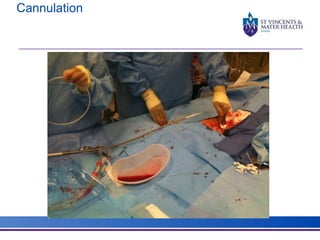
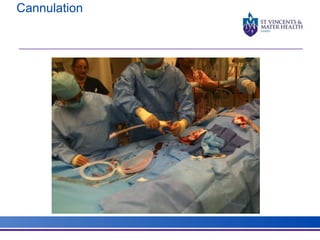
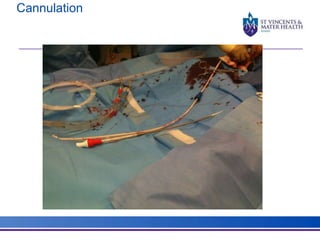
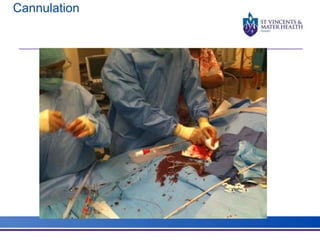
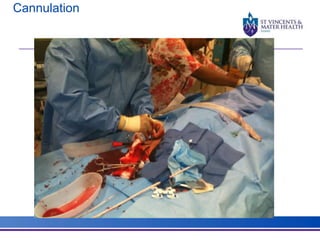
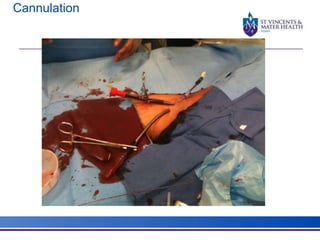
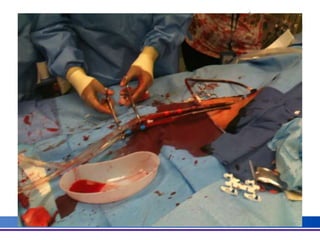
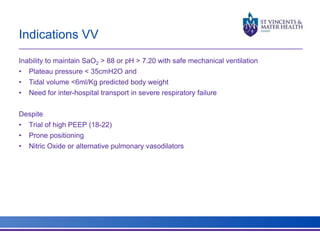
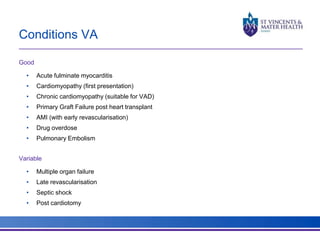
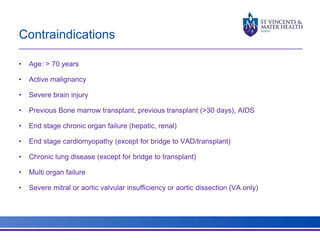
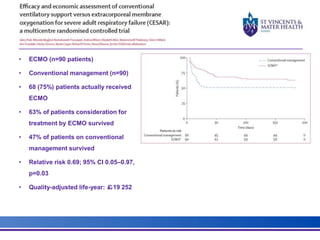
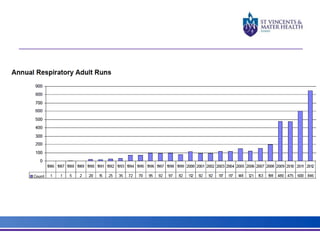
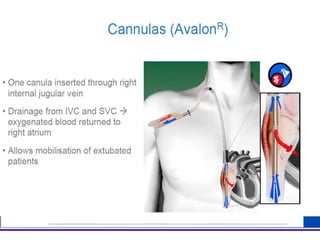
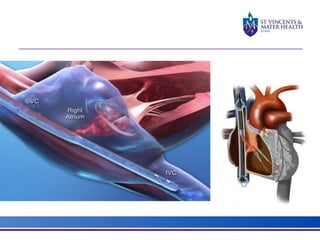
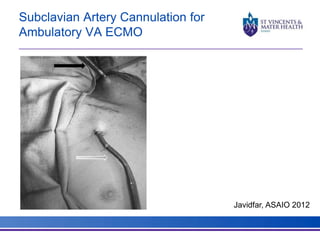
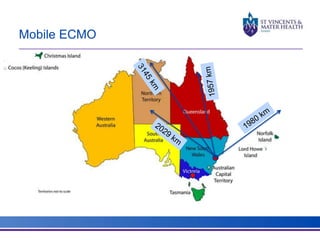
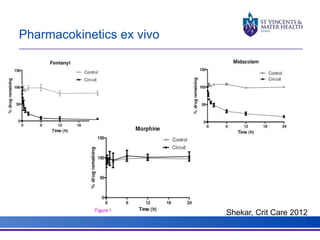
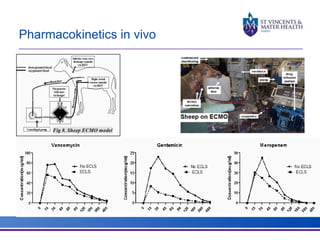
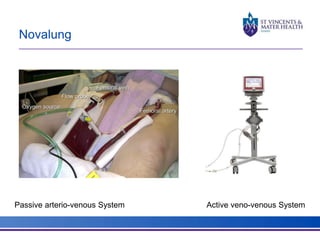
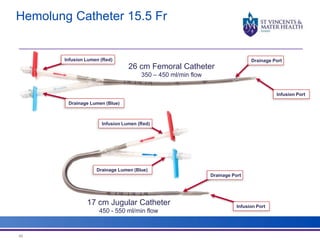
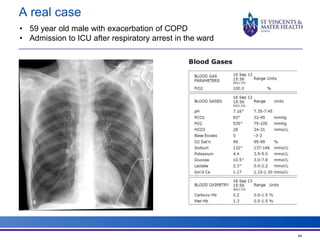
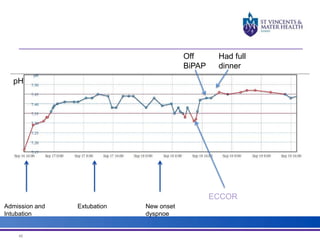
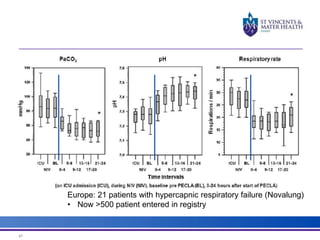
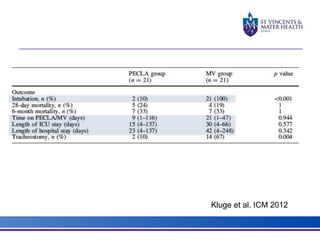
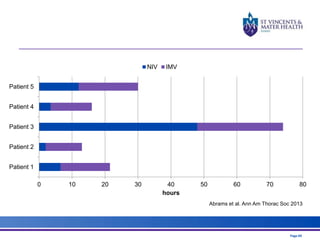
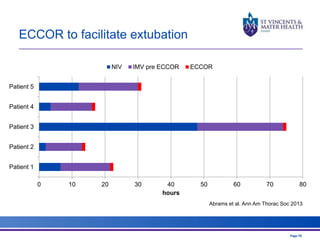
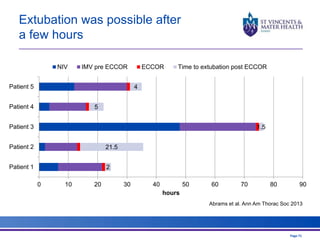
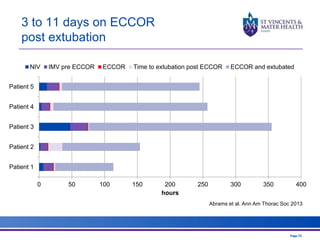
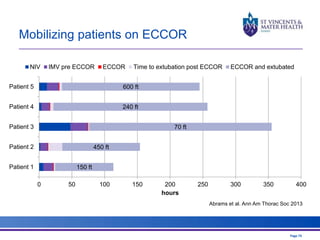
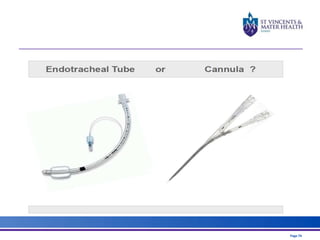
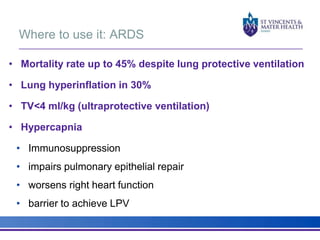
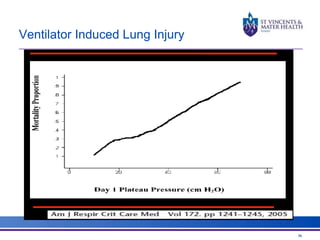
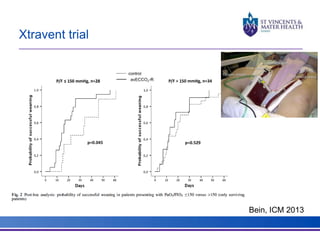
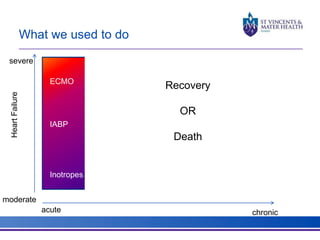
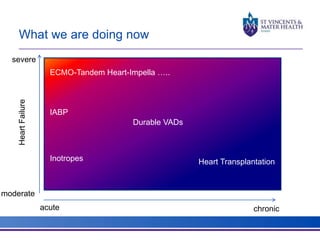
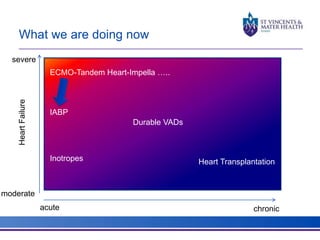
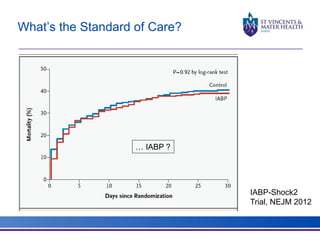
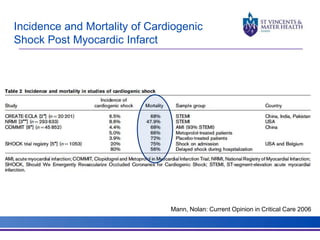
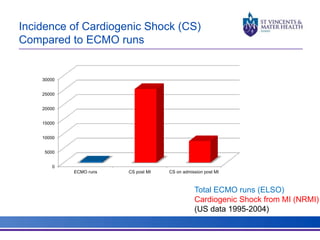
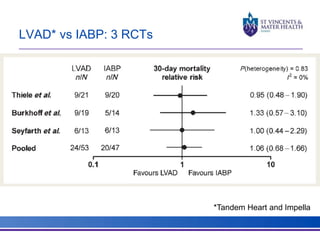
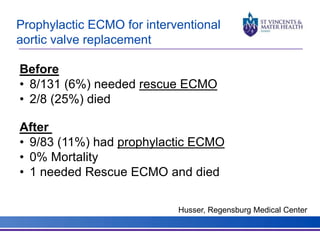
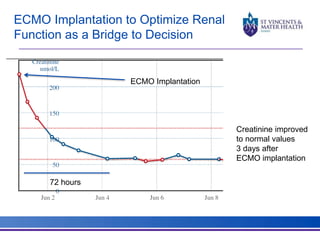
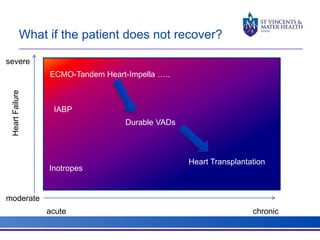
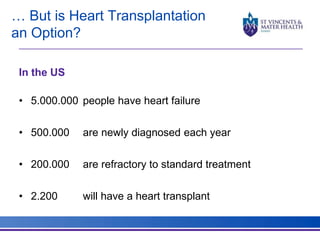
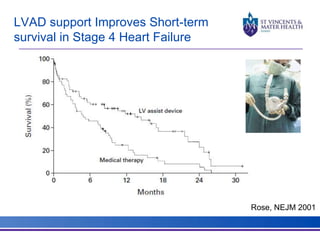
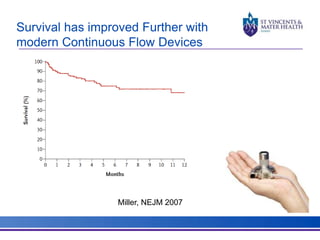
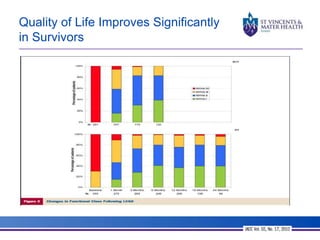
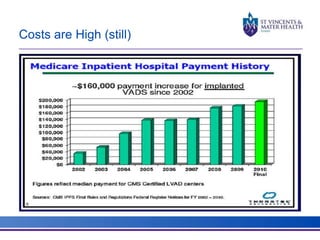
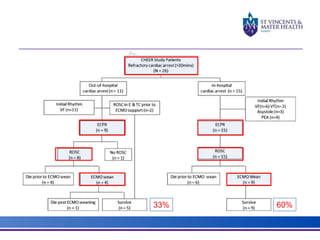
The document discusses advancements in extracorporeal life support (ECLS), particularly focusing on ECMO applications for various patient conditions, including severe acute respiratory distress syndrome and cardiogenic shock. Key points include the criteria for patient selection, indications and contraindications for treatment, and emerging technologies in ECLS. The document emphasizes the need for ongoing research to optimize patient outcomes and ethical considerations in treatment selection.