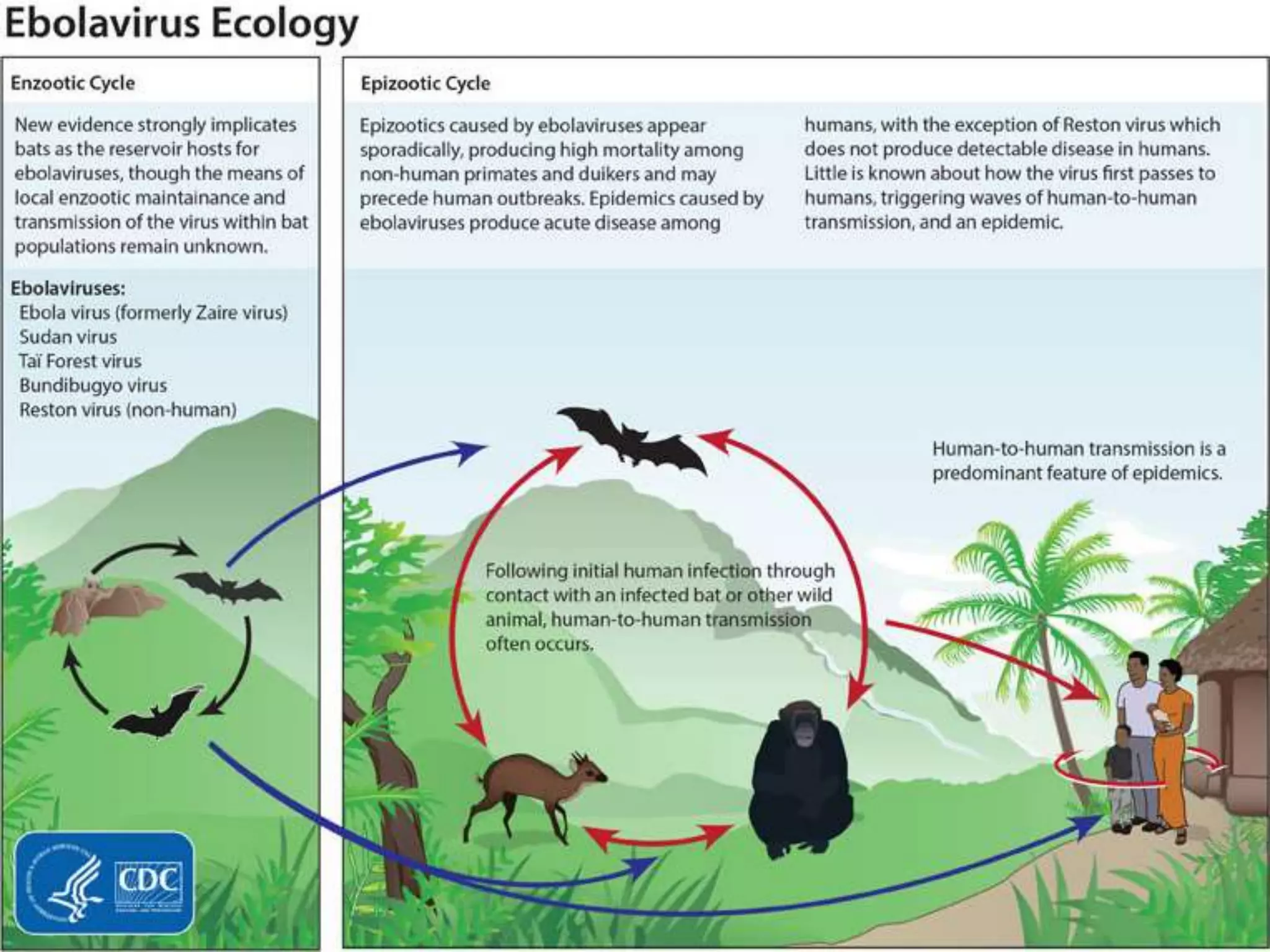
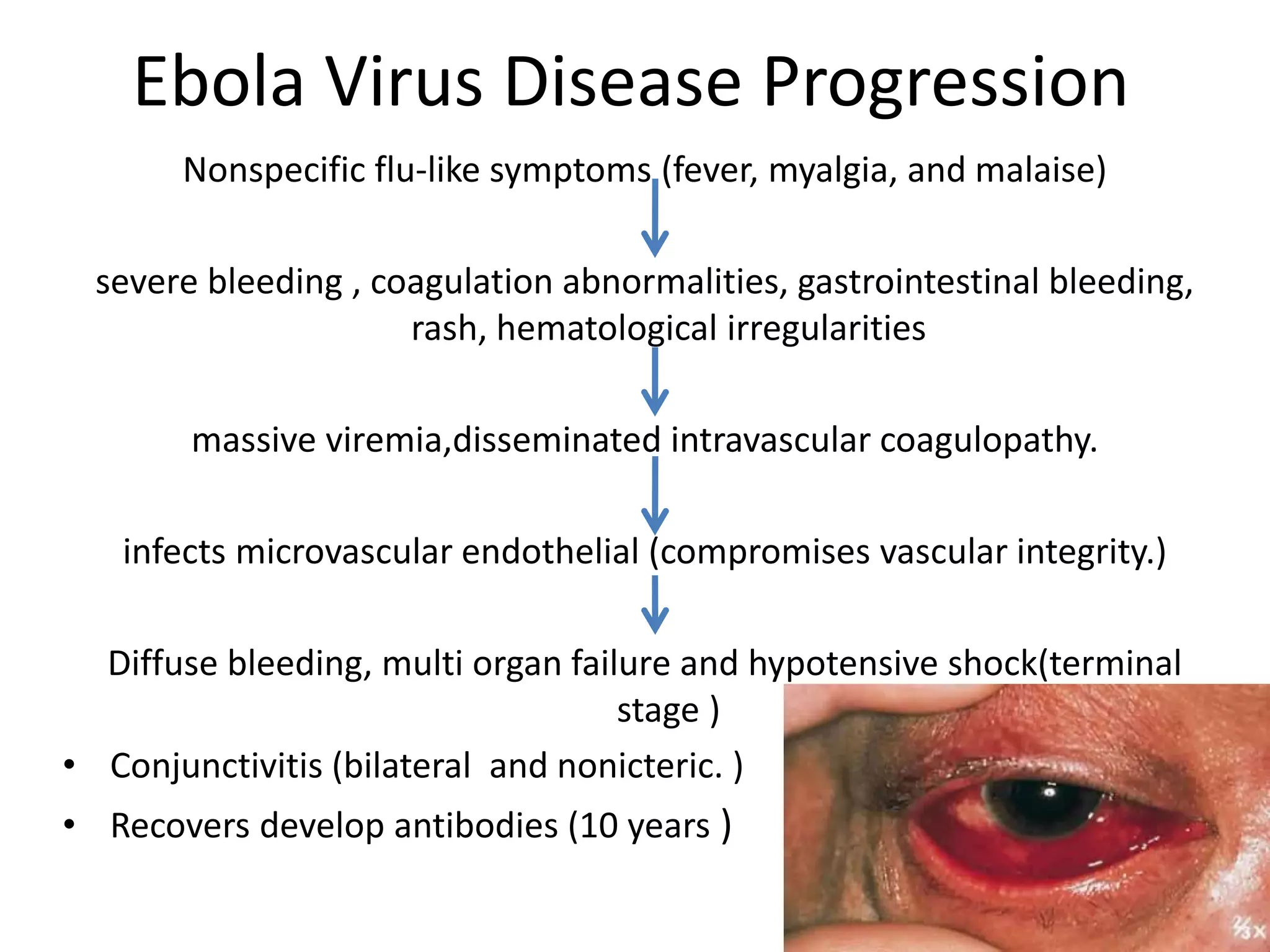
The document provides an extensive overview of Ebola virus, detailing its classification, transmission, symptoms, and outbreaks, along with prevention and treatment efforts. It highlights the significant mortality rates associated with Ebola and discusses the challenges in controlling past outbreaks, particularly in West Africa. Additionally, it addresses the possibility of sexual transmission and the emergence of persistent infections in survivors, raising concerns about future outbreaks.

























![Why this flare-up
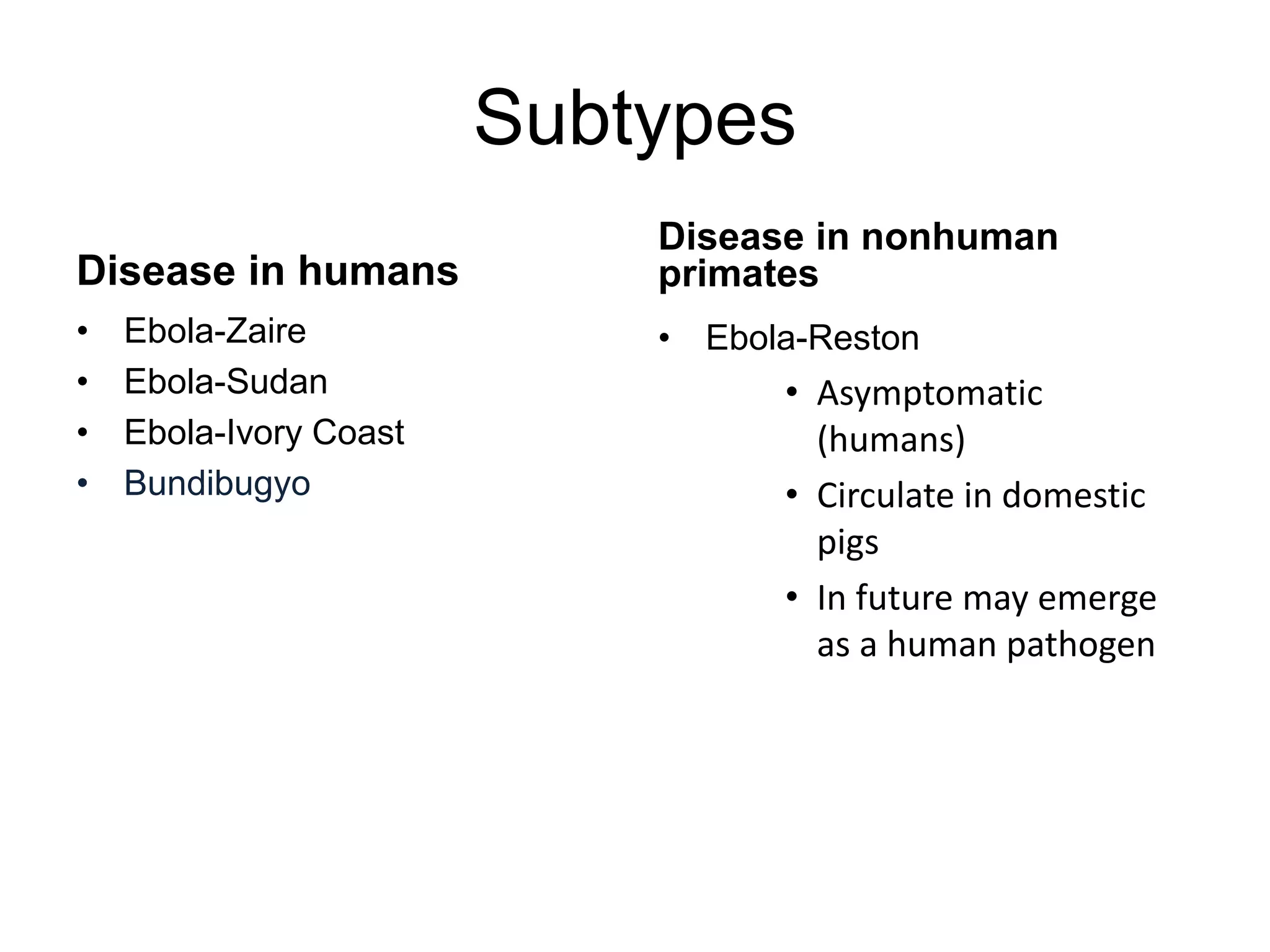
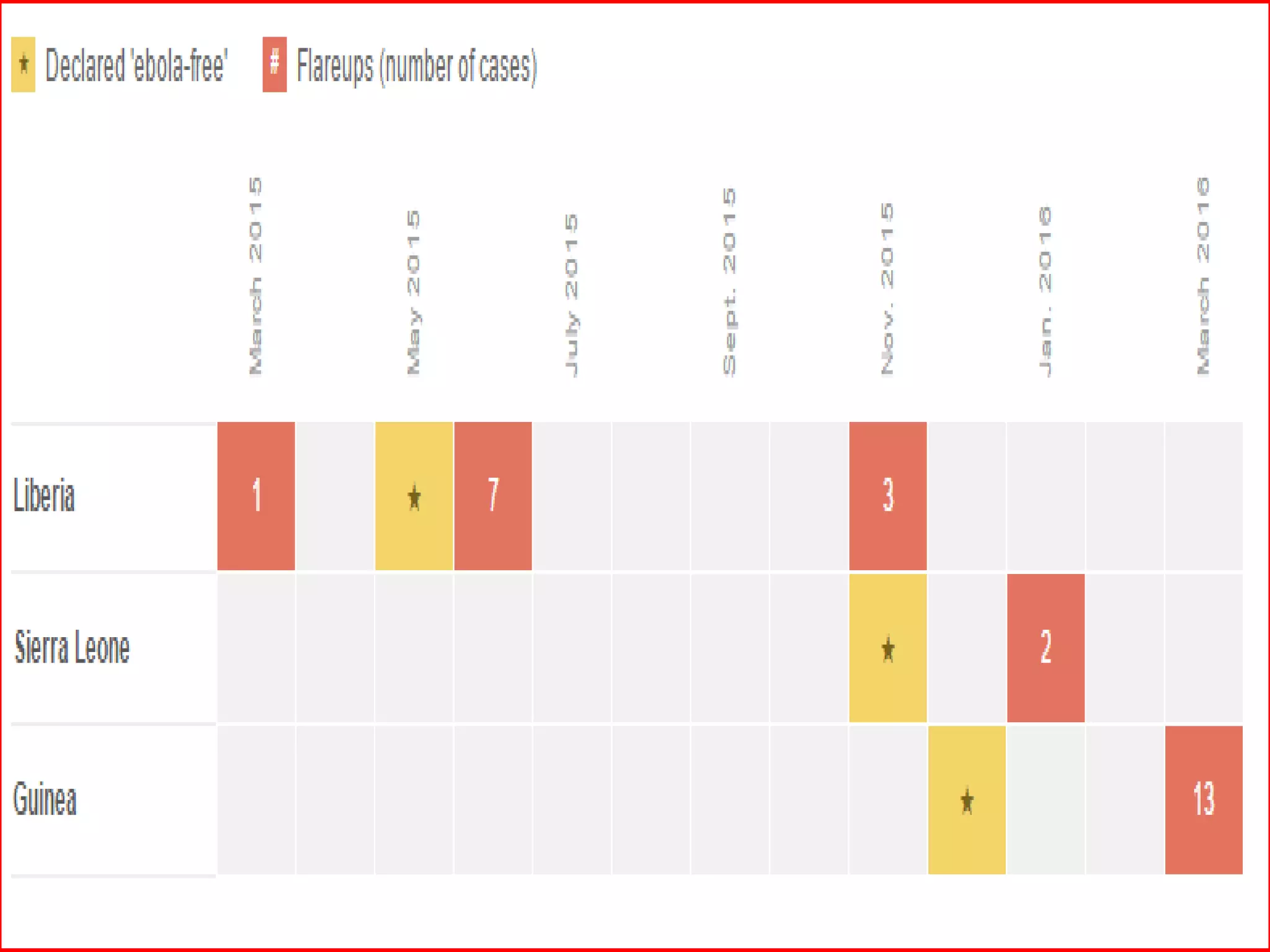
• persistent infection (eyes, spinal fluid, breast milk, testis)
• Sexual transmission is No. 1 concern
• In some cases virus persisted >1 yr
• Out of last 3 flare ups 2 is due to sexual transmission
• What about the other?????? (June 2015 Flare up)
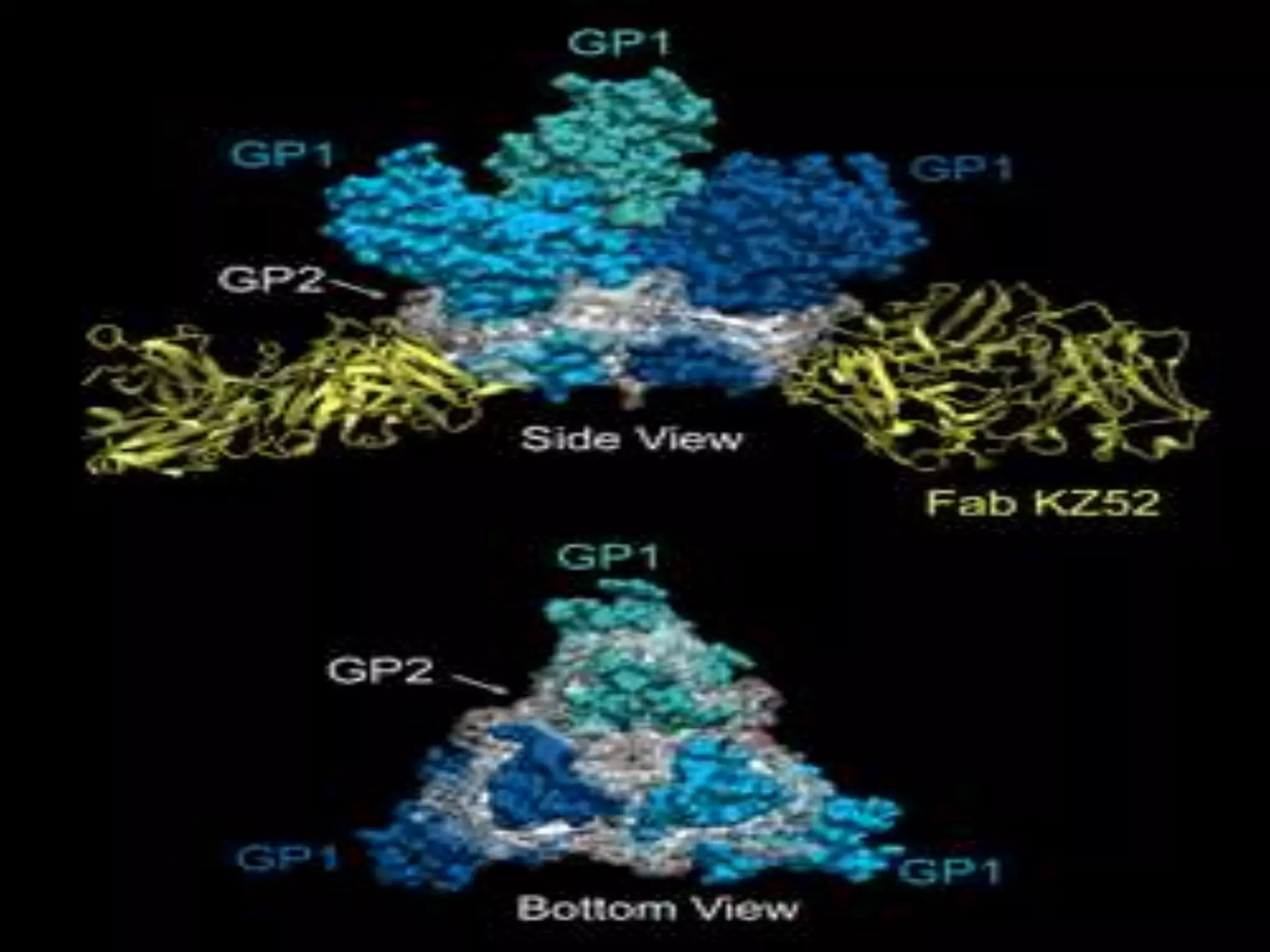
“We believe that most, if not all, the clusters of new Ebola cases have come
from [persistent infections in] survivors, but sometimes it's very hard to
determine that with certainty," -Dr. Thomas Frieden, Director,Centers for
Disease Control and Prevention in the U.S.](https://image.slidesharecdn.com/ebola-161005060458/75/Ebola-and-Its-recent-scenario-37-2048.jpg)



