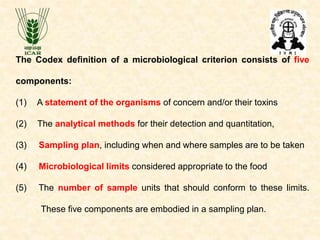
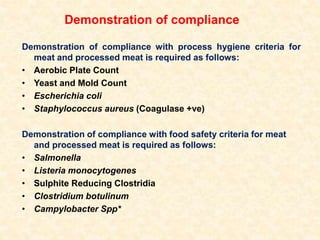
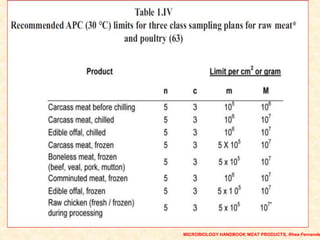
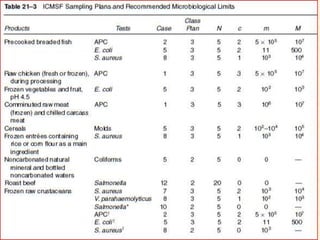
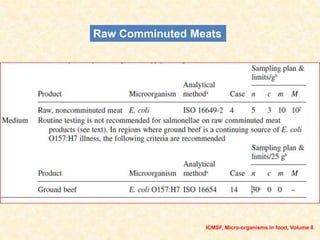
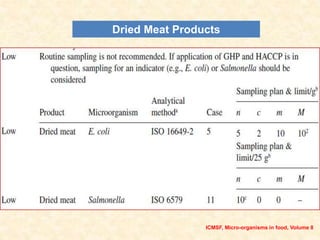
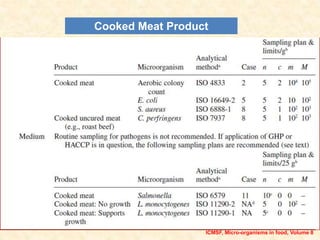
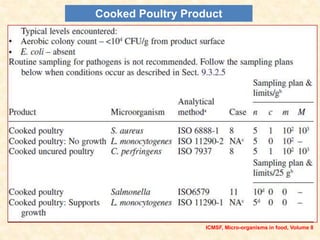
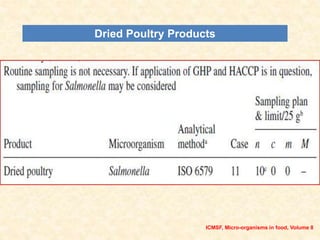
Standards provide technical specifications and criteria to ensure products are fit for purpose. Product standards specify characteristics. Codex defines microbiological criteria as: the organisms of concern, detection methods, sampling plan, appropriate limits, and number of conforming samples. Standards indicate indicator and pathogenic microorganisms of concern for meat. Compliance is demonstrated through aerobic counts, pathogens like Salmonella and Listeria. Sampling plans define requirements and stringency using parameters like sample number, acceptable number of marginal results, and microbiological levels for acceptable and unacceptable quality. Stringency is guided by risk level and intended use.