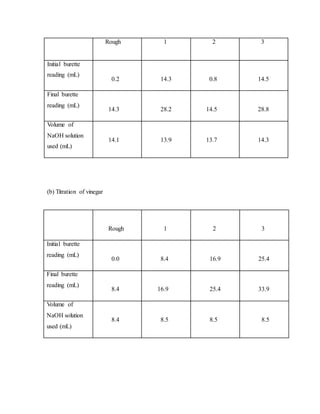
This document describes an experiment to determine the percentage of ethanoic acid in a vinegar sample. Vinegar is made from the oxidation of ethanol and contains acetic acid as its main component. The experiment involves titrating a vinegar sample with a standardized NaOH solution using phenolphthalein as an indicator. Multiple titrations are performed and the data is used to calculate the molarity and concentration of acetic acid in the original vinegar sample. The percentage of acetic acid is determined to be 3.63% w/v.