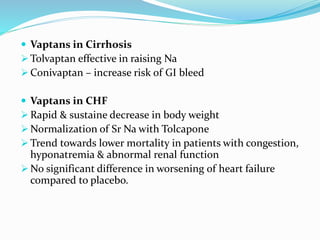
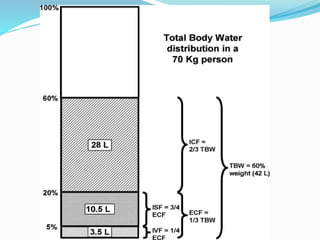
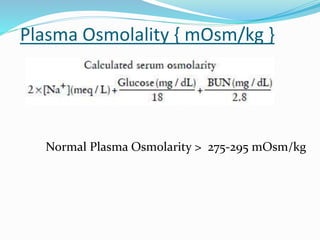
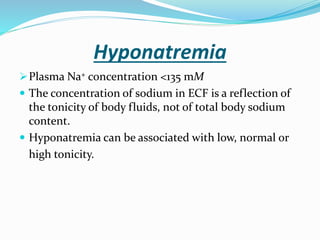
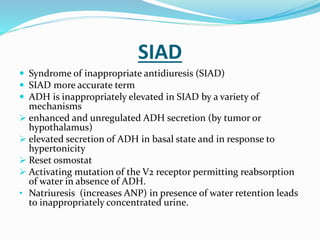
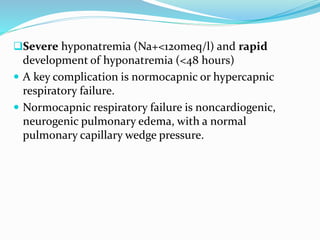
Hyponatremia is the most common electrolyte abnormality seen in hospitalized patients. It is caused by an imbalance of water in the body, resulting in a dilution of sodium concentration. The document discusses the various types of hyponatremia (hypovolemic, euvolemic, hypervolemic) based on extracellular fluid volume status and their underlying causes such as SIADH, heart failure, liver cirrhosis. It also covers the diagnostic evaluation, management principles, and treatment approaches for acute symptomatic and chronic hyponatremia which involves slow correction of sodium levels to avoid osmotic demyelination syndrome.





![Pseudo-hyponatremia > associated with normal or
increased tonicity.
Isotonic hyponatremia
expansion of extracellular fluid with isotonic fluids
that do not contain Na
there is no transcellular shift of water but the [Na+]
decreases
Ex- hypertriglyceridemia
hyperproteinemia( as in Multiple Myeloma)
rise in plasma lipids of 4.6 g/L or plasma protein
concentrations greater than 10 g/dL will decrease the
sodium concentration by approximately 1 mEq/L.](https://image.slidesharecdn.com/myhypo-na-151122090605-lva1-app6891/85/Approach-to-hyponatremia-10-320.jpg)























![ Chronic or slowly developing hyponatremia
0.5 meq/l/h
total 8-12 meq/l/day or
< 10 mMol in 1st 24 hrs, < 18 mMol in 48 hrs
Water Restriction
The urine:plasma electrolyte ratio (urinary [Na+]+[K+]/plasma [Na+]) indicator
of electrolyte-free water excretion
>1 restricted more aggressively (<500 mL/d),
1 restricted to 500–700 mL/d,
<1 restricted to <1 L/d.
In hypokalemic pnts > inj KCl or Pottasium supplements.
By this, generally Na levels are corrected.
Oral salt tablets
oral furosemide 20 mg bd plus oral salt tablets
Demeclocycline 600 to 1200 mg/day
Vaptans](https://image.slidesharecdn.com/myhypo-na-151122090605-lva1-app6891/85/Approach-to-hyponatremia-36-320.jpg)
![ Equations are available to help calculate the initial rate of
fluids to be administered.
A widely used formula is the Adrogue-Madias formula.
Change in serum Na+ with infusing solution=
[infusate (Na + K)]-serum Na
(total body water +1)
Infusate Na+ is the [Na+] in the infused fluid (154meq/l in
0.9%NS, 513meq/l in 3%NS, 77meq/l in 0.45%NS & 0
meq/l in D5W).
The above equation predicts the amount of [Na+] change
by 1 liter of infusate.
Dividing the targeted change in Sr Na by the result of above
equation gives volume of infusate required & thus the rate
of infusion.](https://image.slidesharecdn.com/myhypo-na-151122090605-lva1-app6891/85/Approach-to-hyponatremia-37-320.jpg)