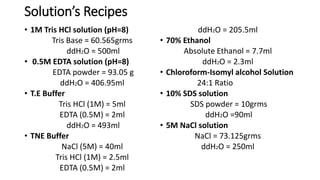
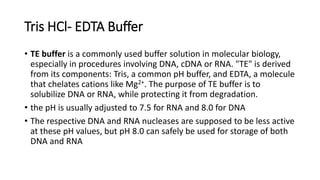
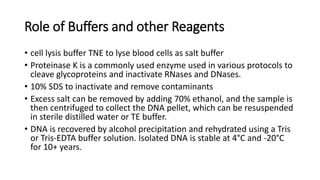
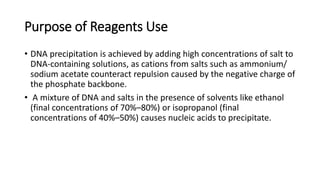
This document describes the process of extracting DNA from human blood. It involves lysing blood cells, digesting proteins, washing red blood cells, precipitating proteins and DNA, and purifying the extracted DNA. The key steps are cell lysis using TNE buffer, protein digestion with Proteinase K and SDS, phenol-chloroform extraction to separate DNA from other cell components, precipitation of DNA with isopropanol, and resuspension of purified DNA in TE buffer. A variety of reagents are required including Tris, EDTA, NaCl, SDS, Proteinase K, phenol, chloroform, isopropanol, and ethanol.