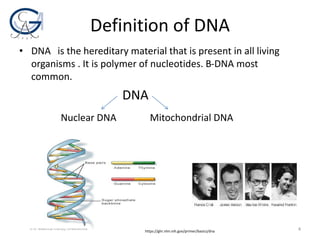
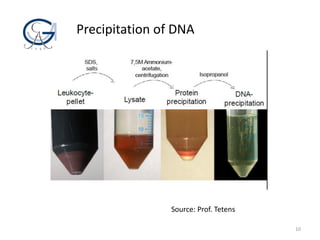
The document provides an overview of DNA isolation, detailing its aims, definitions, purposes, sample selection, and the basic steps involved in the process. Key points include the chemistry behind cell lysis, precipitation methods for DNA extraction, and the importance of sample type for successful DNA recovery. It highlights both scientific and forensic applications of DNA isolation, emphasizing its relevance in diagnostics and identification.