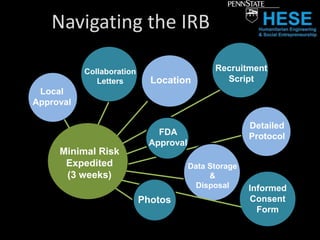
This document discusses ethical challenges in conducting research with developing communities. It provides an overview of student ventures conducting applied science and design research projects in places like Kenya. The importance of research for these projects is described as well as navigating the institutional review board (IRB) process for approving research involving human subjects. Some of the challenges discussed include obtaining local approvals, addressing informed consent and risk when working in unfamiliar contexts, incentives for research participation, and balancing the discipline of research with chaotic real-world conditions in developing areas. The document concludes with lessons learned from a student research trip to Kenya regarding preparing protocols early, managing risks, addressing language barriers, and determining criteria for community engagement and participation.