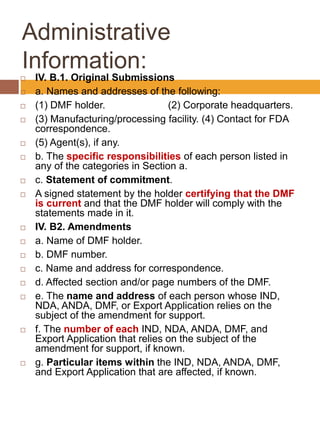
A Drug Master File (DMF) is a confidential submission to the FDA containing detailed information about facilities, processes, or materials used in manufacturing drugs. There are five types of DMFs covering manufacturing sites, drug substances, packaging materials, excipients, and other reference information. A DMF supports but is not a substitute for applications to conduct clinical trials or market a drug. It is kept confidential but reviewed when its information is cited in an application. DMFs must be submitted in English and include administrative information, contents according to the type of information, and stability and other data as applicable guidelines require.