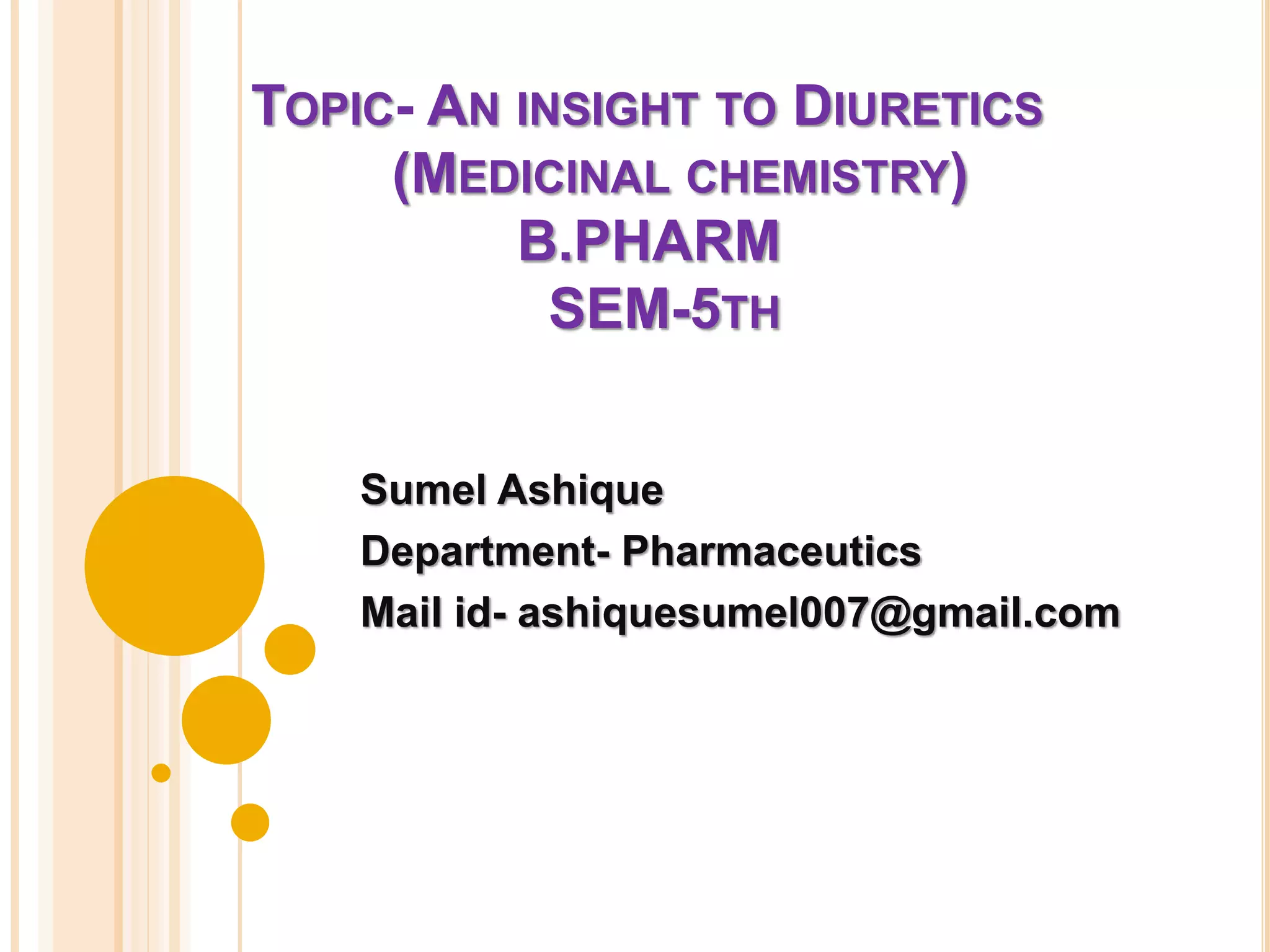
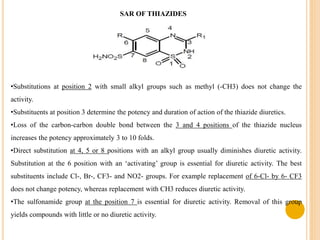
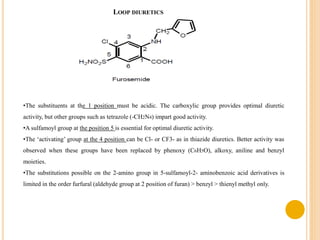
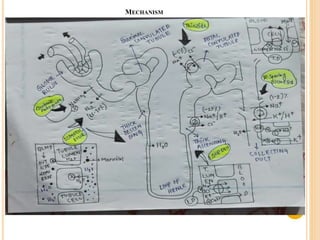
This document provides an overview of diuretics including their definition, classification, mechanisms of action, structure-activity relationships, and examples of conditions they are used to treat. Diuretics work by reducing sodium reabsorption at different sites in the nephron. They are classified as osmotic, loop, thiazide, potassium-sparing, or carbonic anhydrase inhibitors. Structure-activity relationships indicate certain chemical groups and substitutions impact diuretic potency. Common uses of diuretics include treating high blood pressure, heart failure, and reducing fluid buildup.