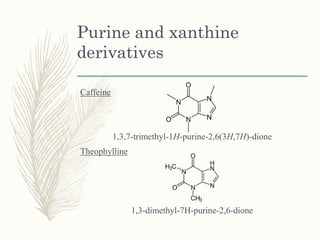
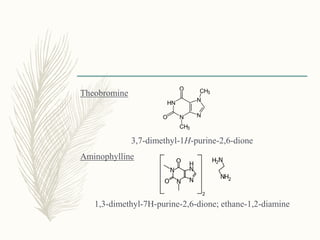
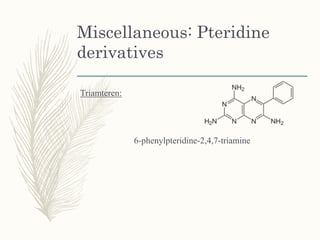
This document provides information on diuretics, including what they are, the conditions they can treat, their mechanisms of action, and classifications. The main points are:
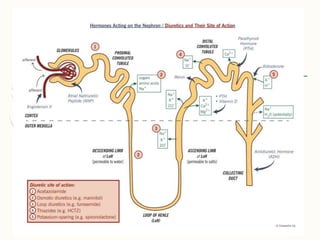
1. Diuretics promote urine production and can treat conditions like heart failure, kidney issues, hypertension, and liver cirrhosis. They work by either increasing glomerular filtration or decreasing tubular reabsorption of water and ions in the kidneys.
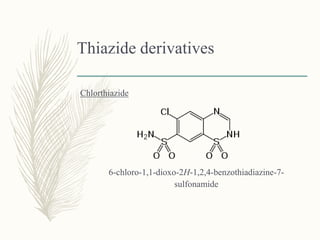
2. Diuretics can be classified as mercurial or non-mercurial. Common non-mercurial classes include thiazides, carbonic anhydrase inhibitors, and loop diuretics which act at different parts of the nephron.
3. Thiaz







![Meralluride
[3-[3-(3-Carboxypropionic acid) ureido]-2-methoxypropyl]-
hydroxy-mercury mixture with theophylline
– Meralluride is employed for the treatment of oedema
secondary to congestive heart failure, the nephrotic state
of glomerulonephritis and hepatic cirrhosis.](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-10-320.jpg)
![Merethoxylline Procaine
– The procaine merethoxylline is an equimolar mixture of
procaine and merethoxylline
– The latter being the inner salt of [o-[[3-(hydroxymercuri)-
2-(2-methoxyethoxy)-propyl]-carbamoyl] phenoxy] acetic
acid.
– It has been used effectively in the treatment of oedema
and ascites in cardiac failure and also in ascites due to
cirrhosis of the liver. The procaine component helps in
reducing the discomfort of local irritation which may be
caused by the mercurial compound when injected into
tissues.](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-11-320.jpg)





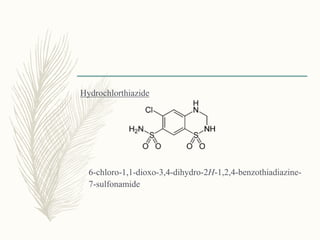

![Benzthiazide
6-chloro-1,1-dioxo-3-(phenylmethylsulfanylmethyl)- 4H-
benzo[e][1,2,4]thiadiazine-7-sulfonamide
It is used as a diuretic and an antihypertensive agent with
pharmacological actions similar to those of hlorothiazide.](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-23-320.jpg)
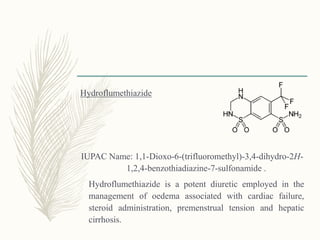
![Cyclothiazide
3-(bicyclo[2.2.1]hept-5-en-2-yl)-6-chloro-3,4-dihydro-2H-
1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
It possesses both diuretic and antihypertensive actions. It is
often used as an adjunct to other antihypertensive drugs,
such as reserpine and the ganglionic blocking agents.](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-24-320.jpg)
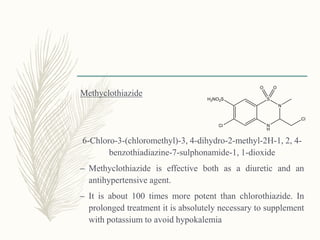
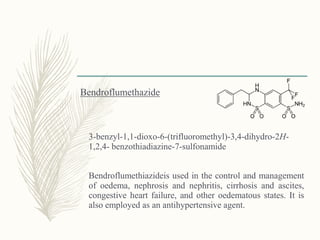
![Cyclopentthiazide
6-chloro-3-(cyclopentylmethyl)-1,1-dioxo-3,4-dihydro-2H-
benzo[1,2,4]thiadiazine-7-sulfonamide
Uses similar to chlorthiazide](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-26-320.jpg)
![Trichloromethiazide
6-chloro-3-(dichloromethyl)-1,1-dioxo-
3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-
7-sulfonamide
Trichlormethiazide belongs to the class of long-acting
diuretic and antihypertensive thiazide.](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-27-320.jpg)
![Polythiazide
6-chloro-2-methyl-3-{[(2,2,2-trifluoroethyl)thio]methyl}-3,4-
dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-
dioxide
Polythiazideis a potent long-acting diuretic and anti
hypertensive agent.](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-28-320.jpg)
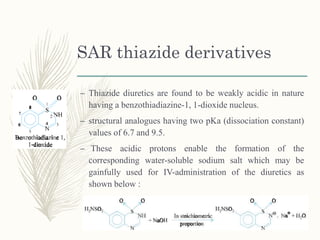



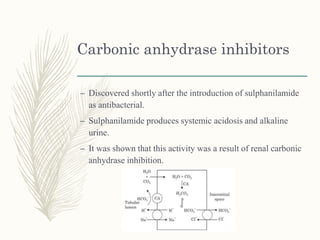

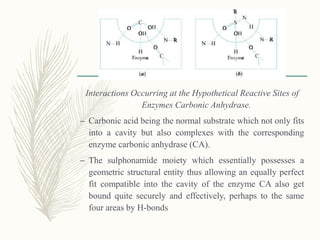
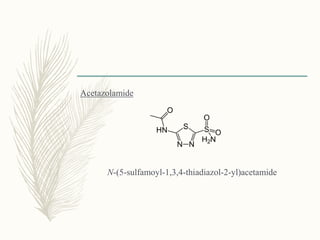
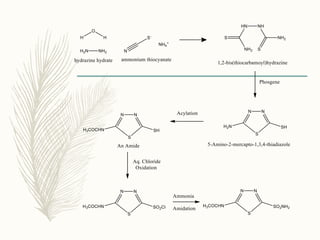

![Methazolamide
N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-
ylidene]acetamide
– Its actions and uses are similar to those of acetazolamide.
– However, its action has been found to be relatively less
prompt but of definitely longer duration than that of the
latter, lasting for 10 to 18 hours](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-39-320.jpg)
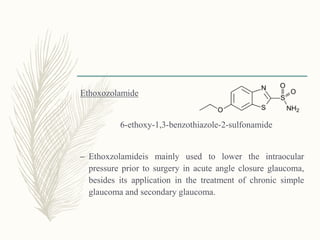


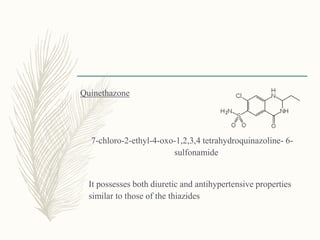


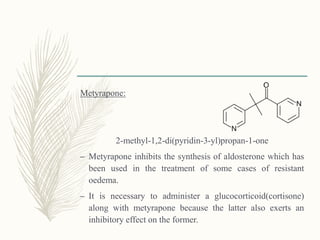

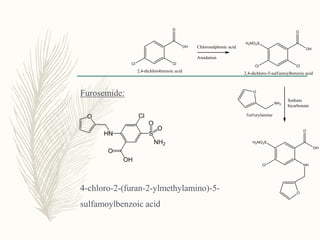


![Phenoxyacetic acid
derivatives
Ethacrynic acid:
[2,3-dichloro-4-(2-methylene
butanoyl)phenoxy]acetic acid](https://image.slidesharecdn.com/diuretics-181229051611/85/Diuretics-55-320.jpg)