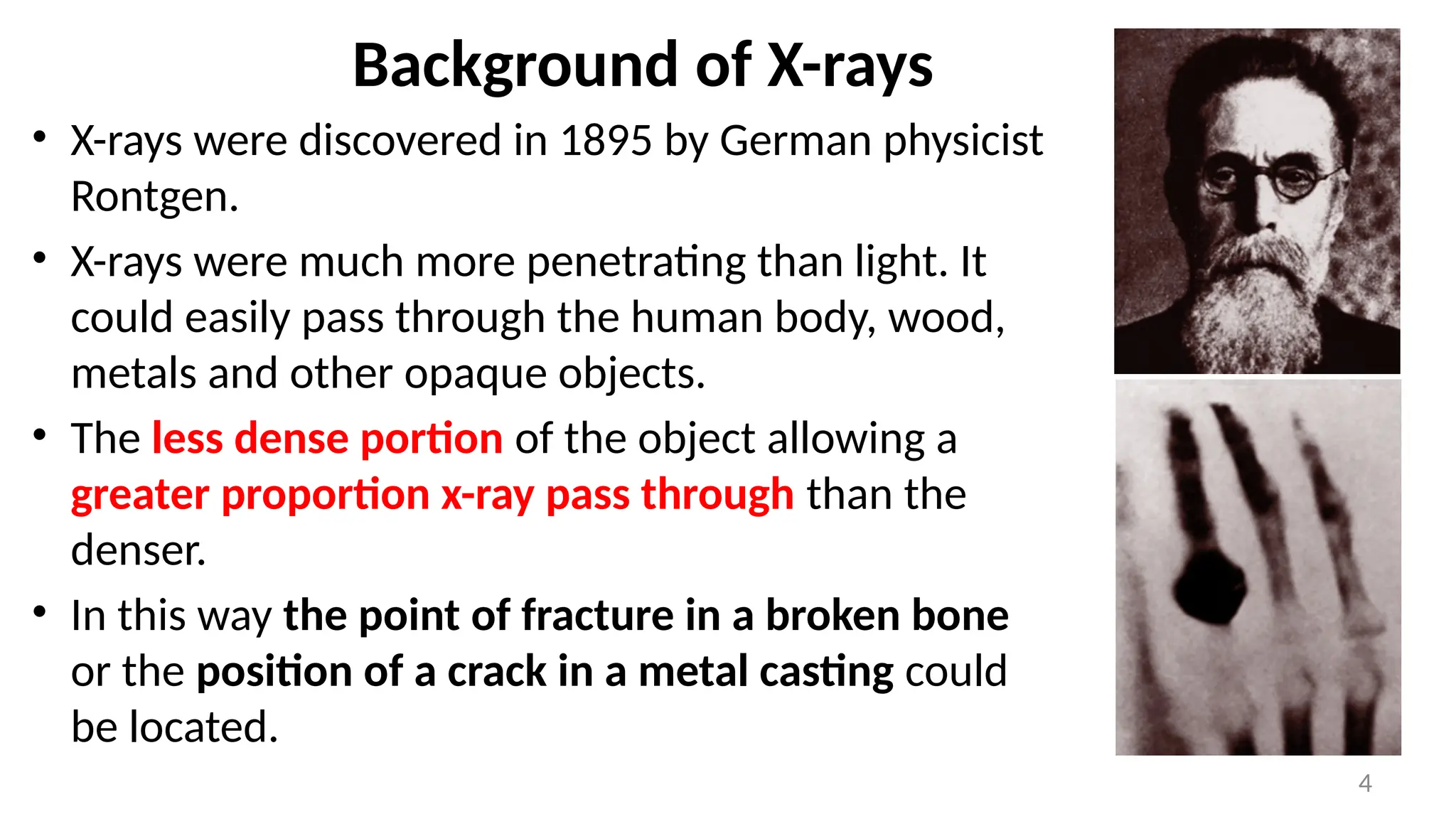
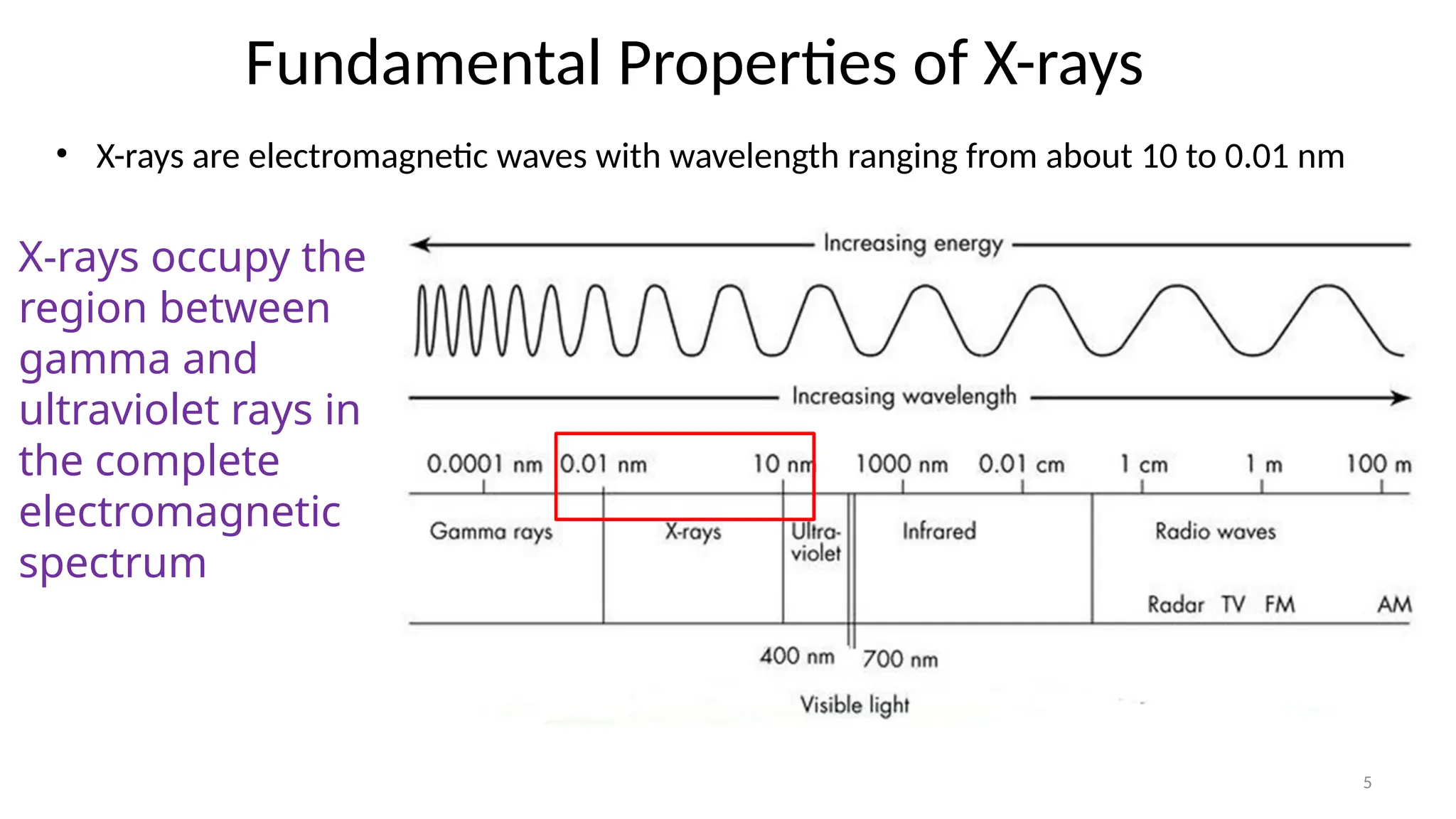
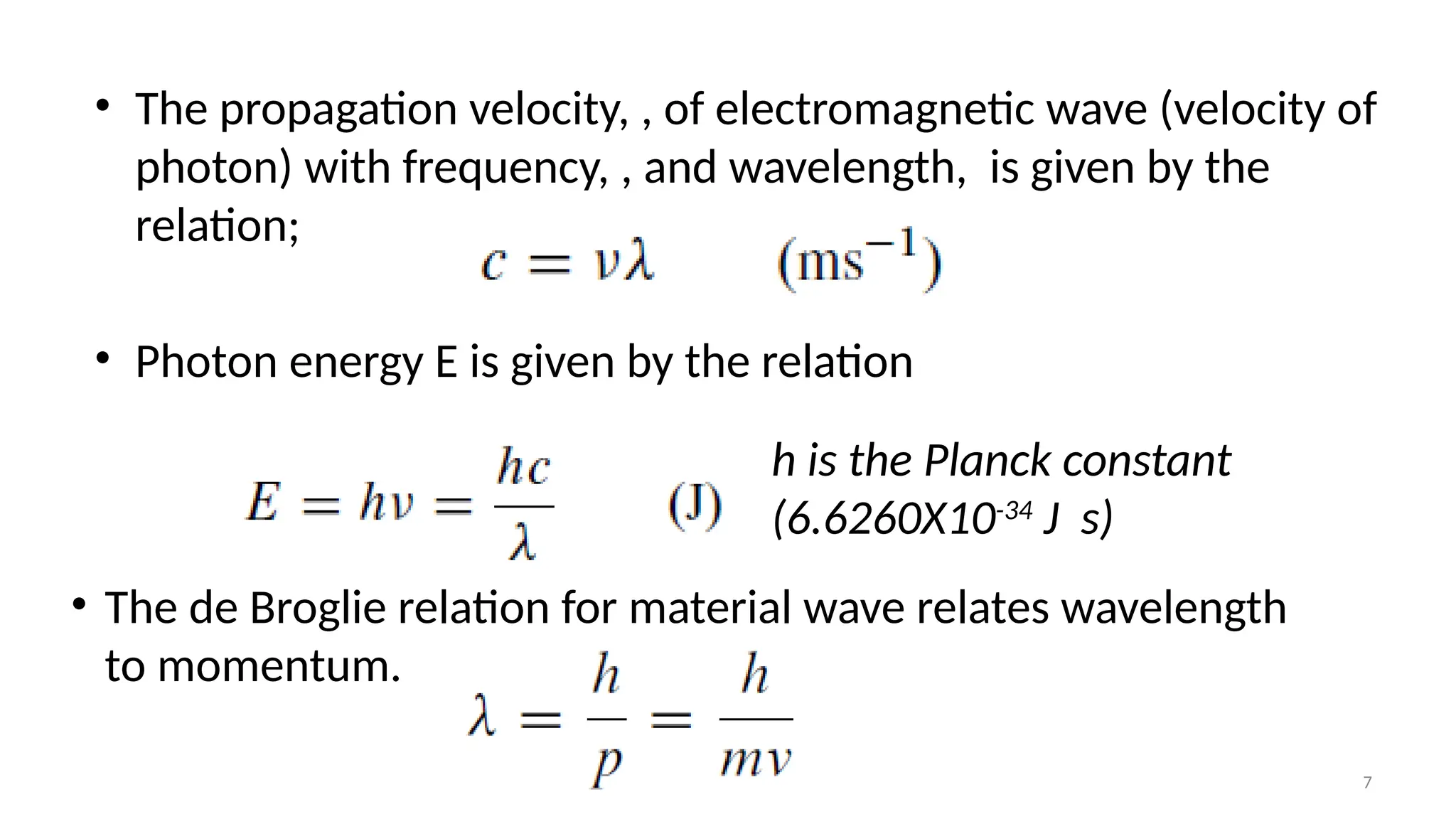
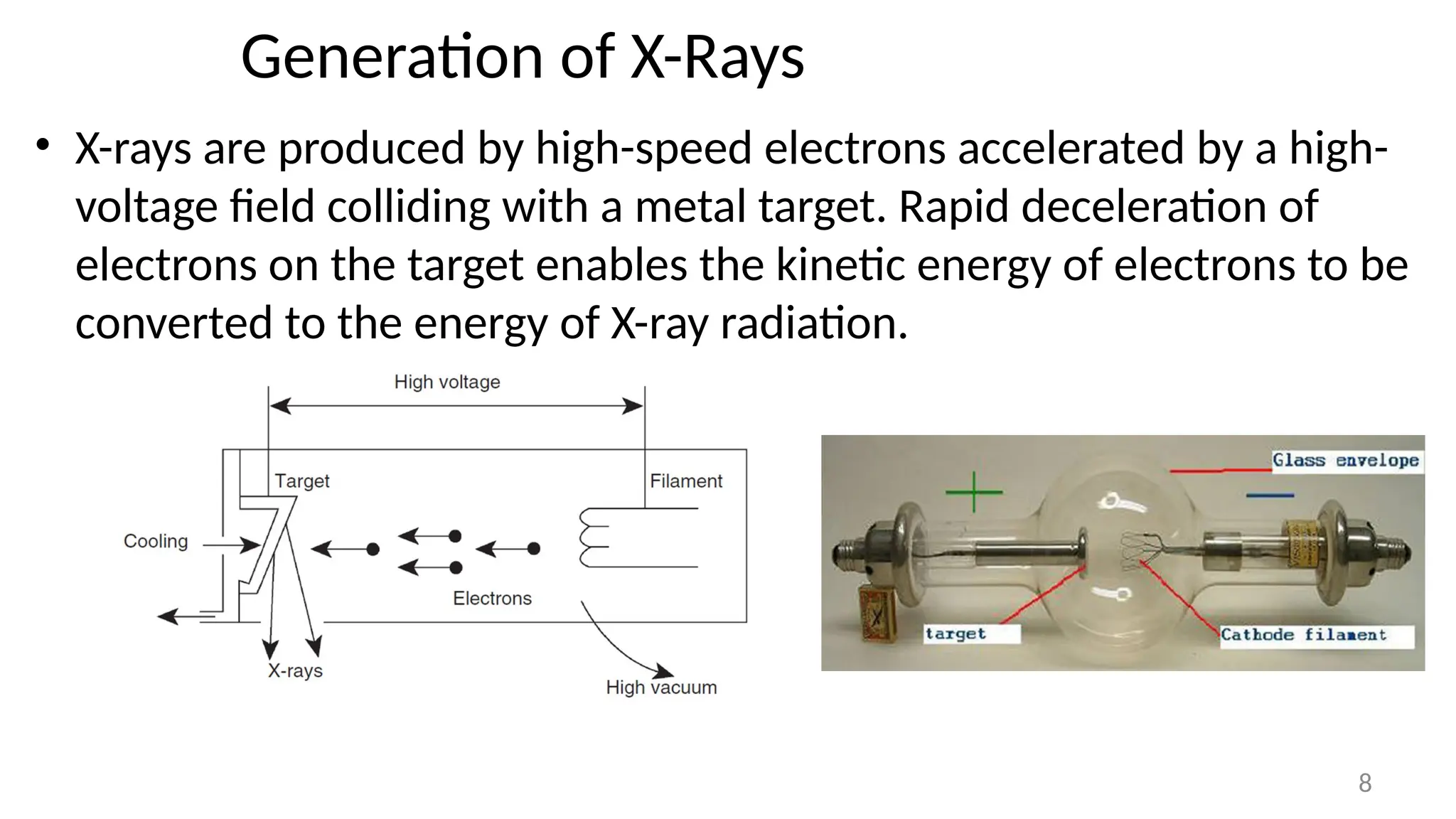
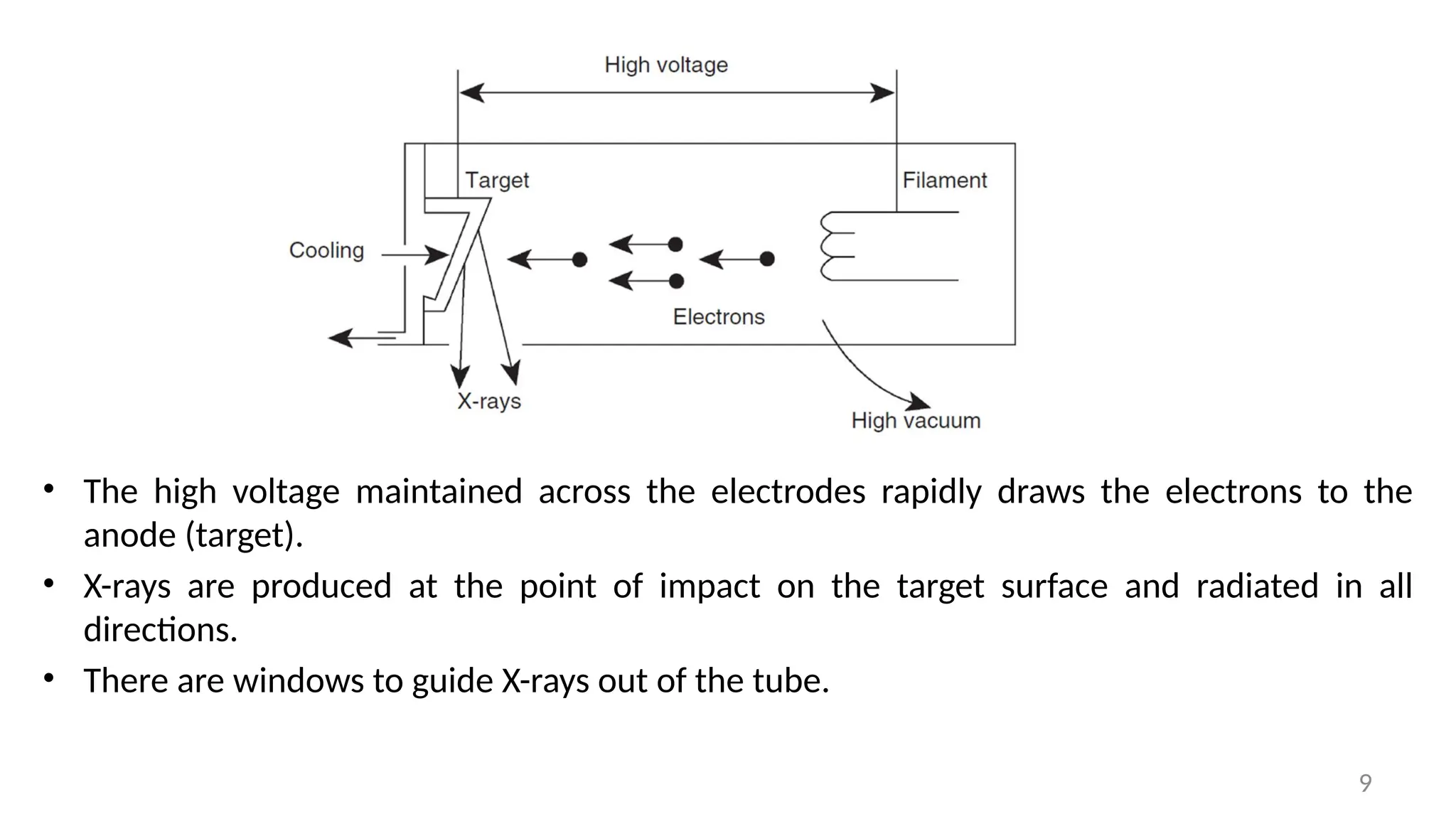
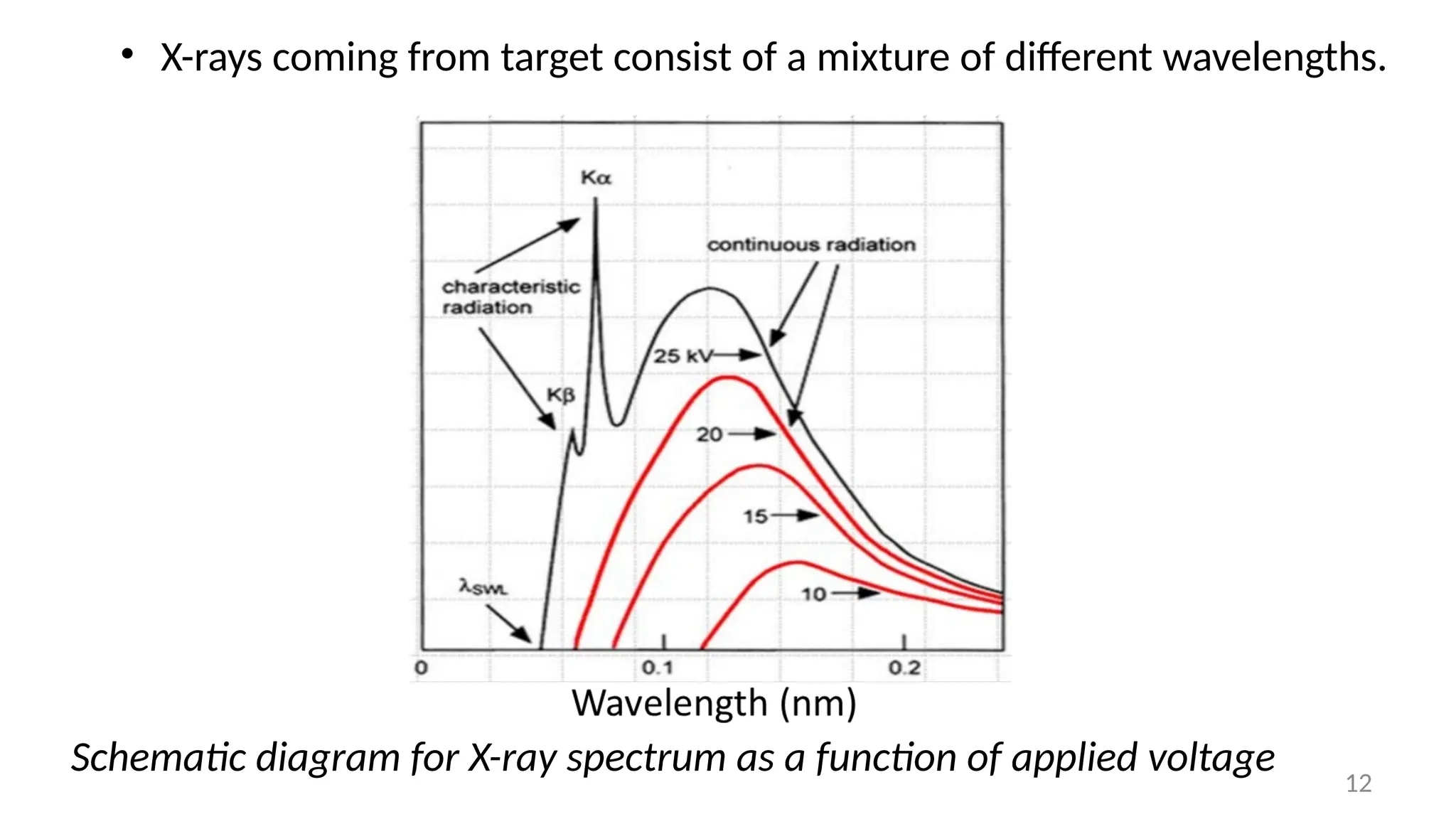
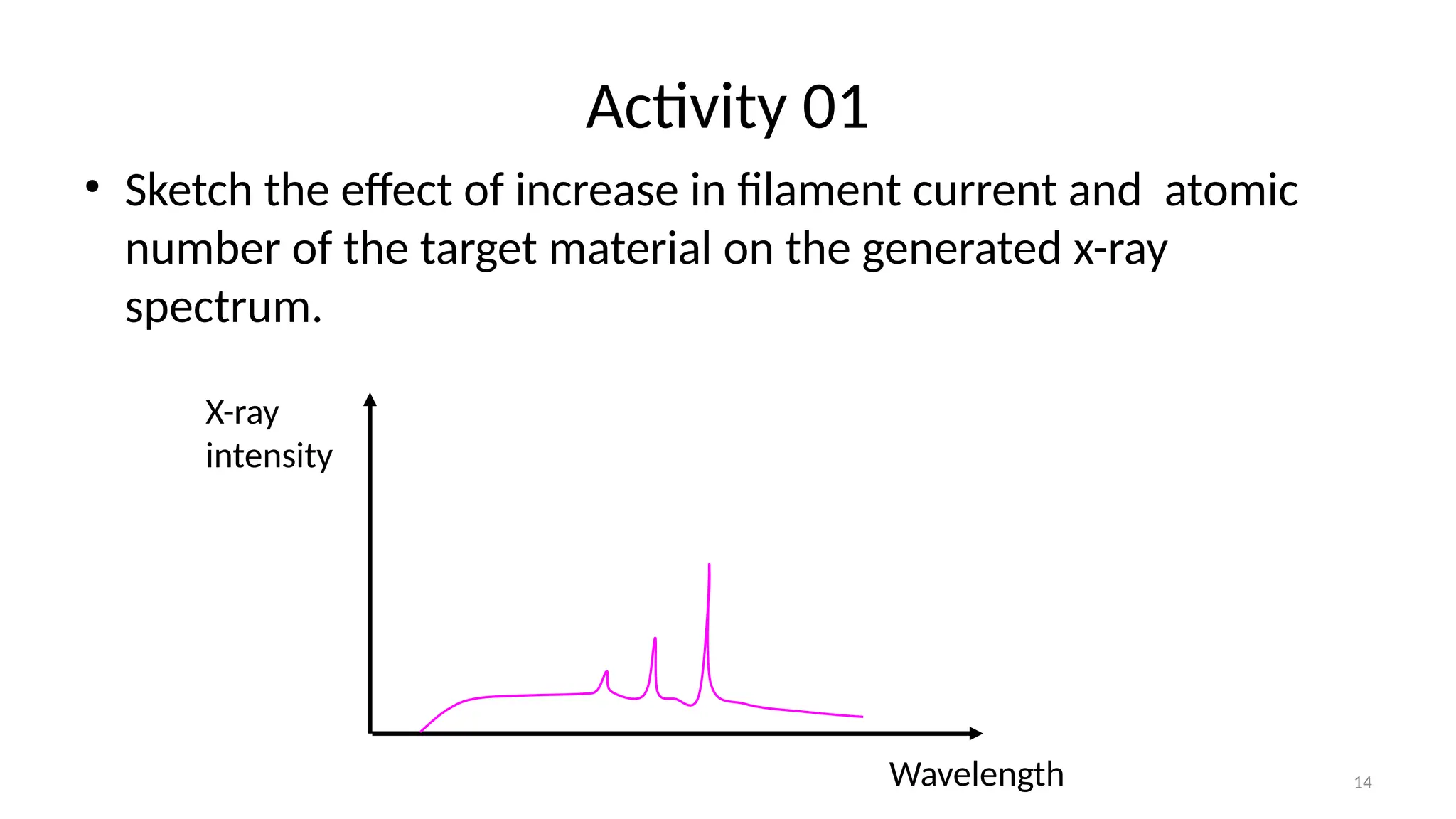
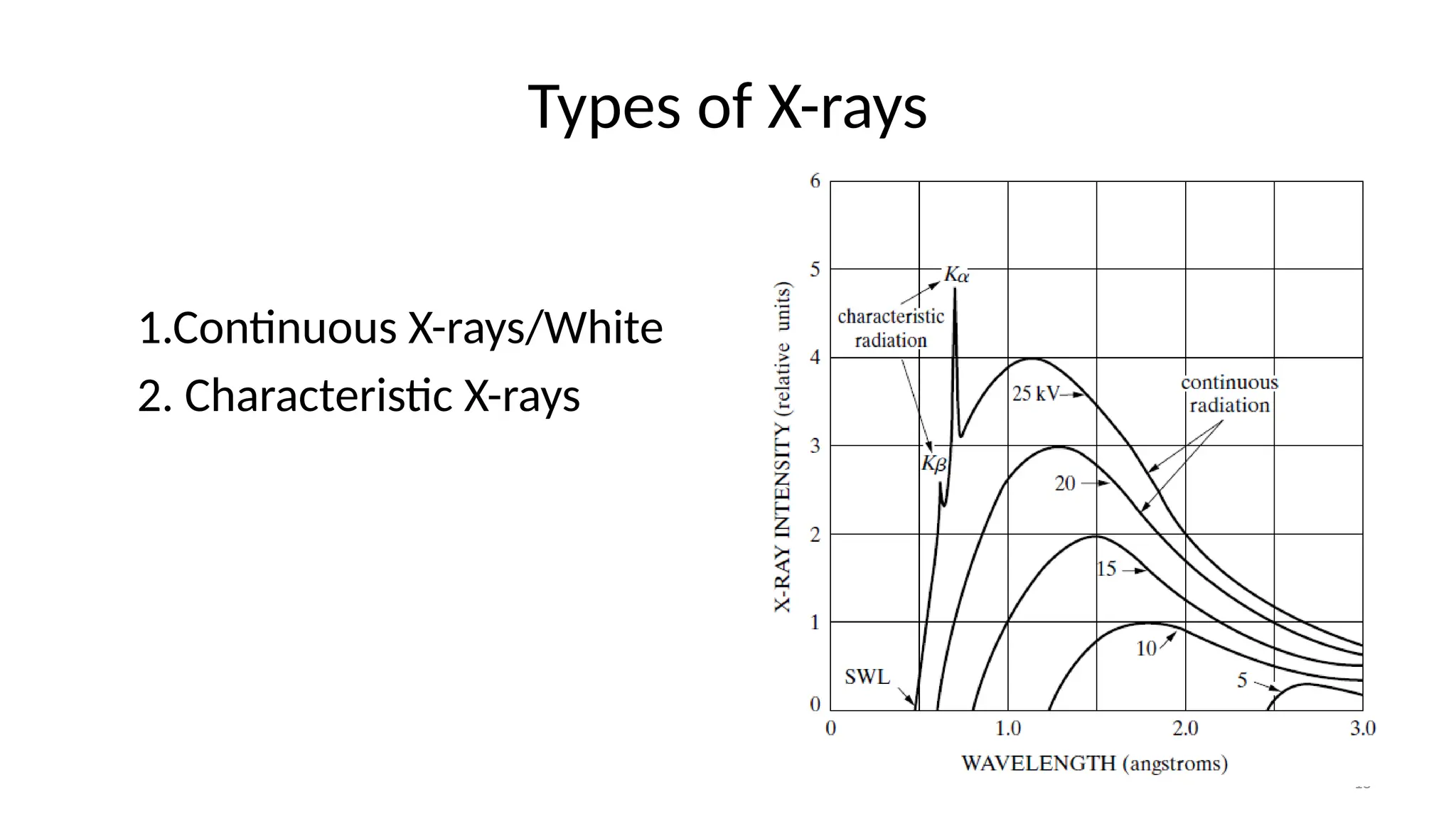
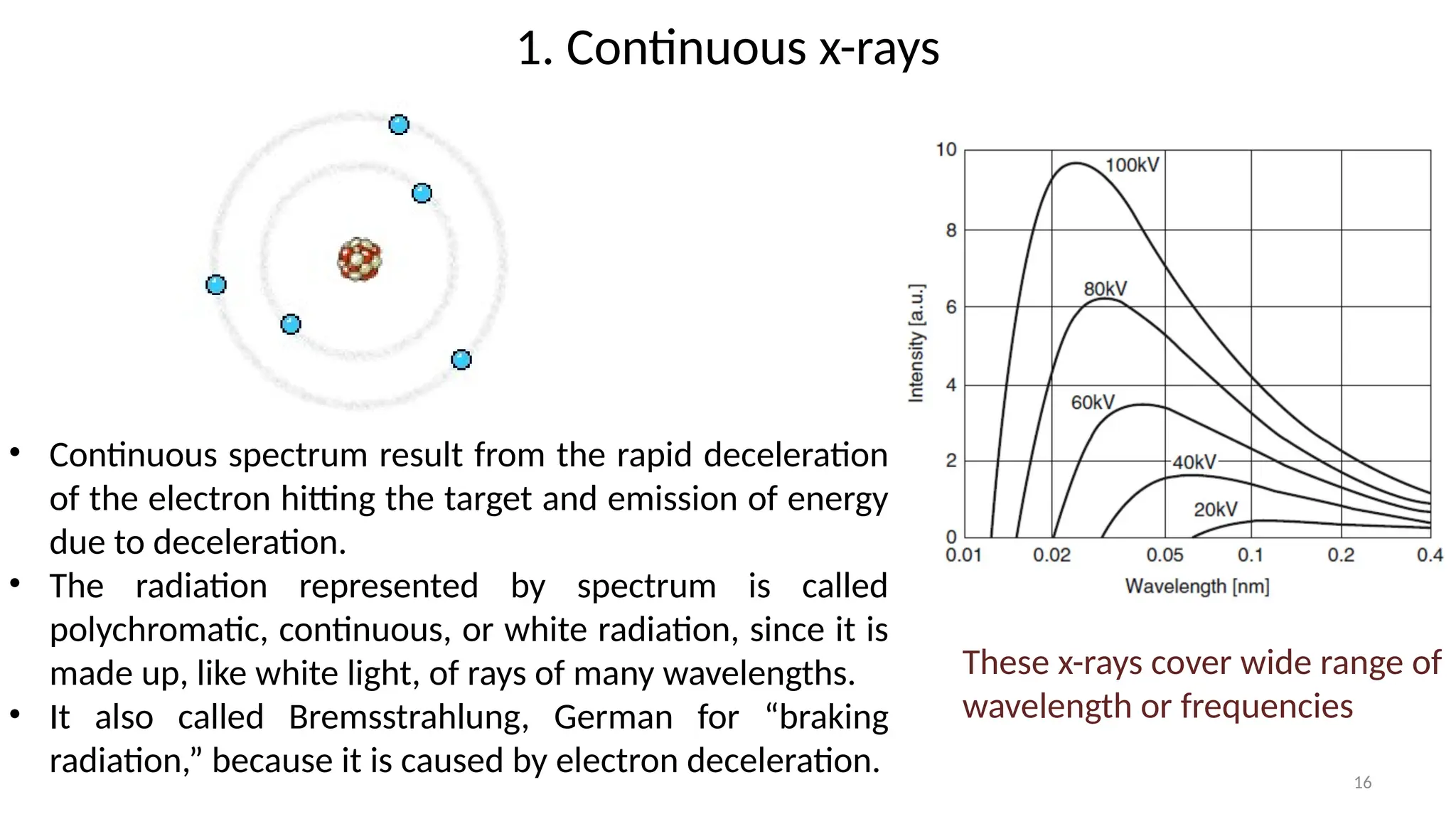
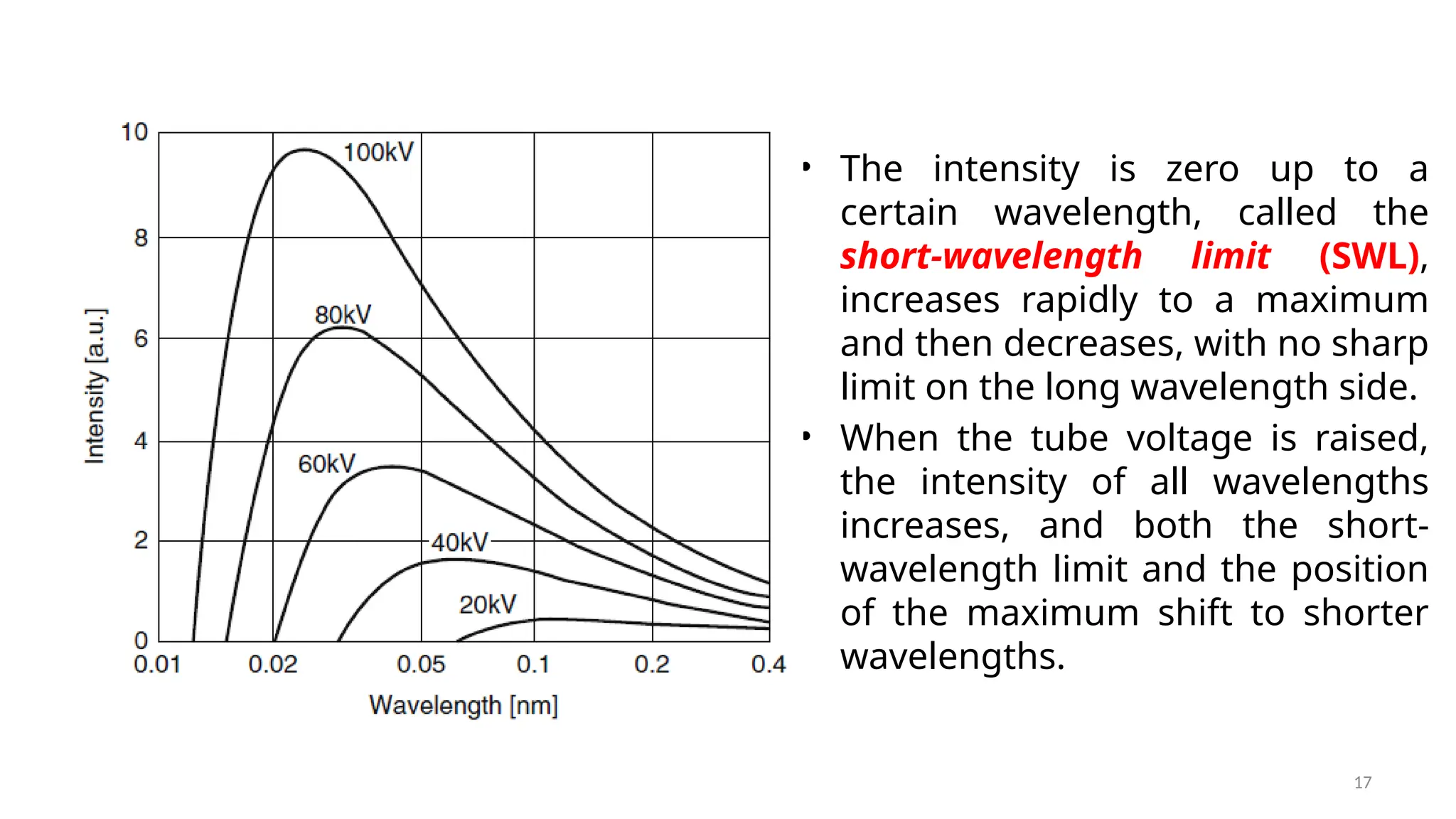
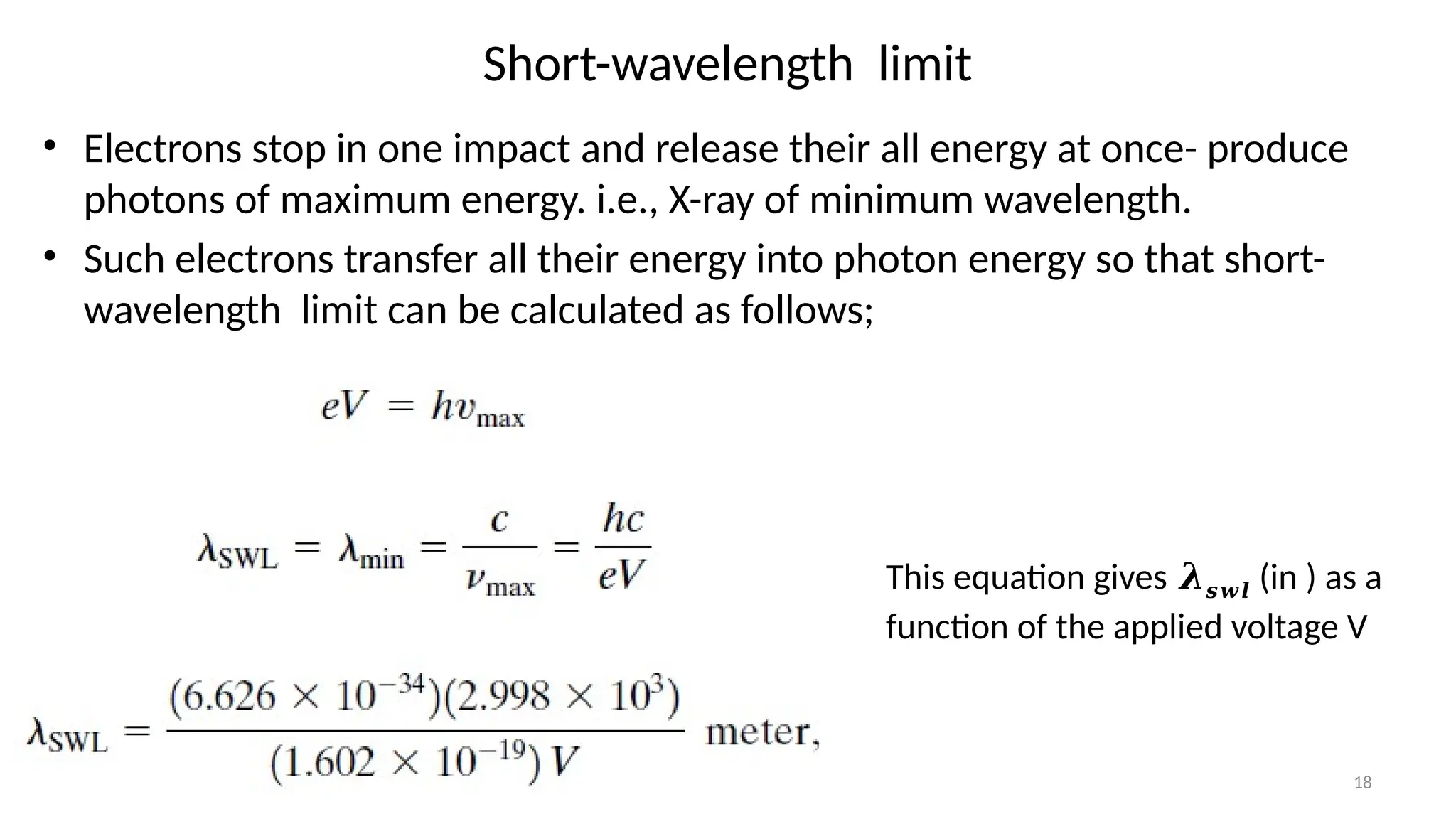
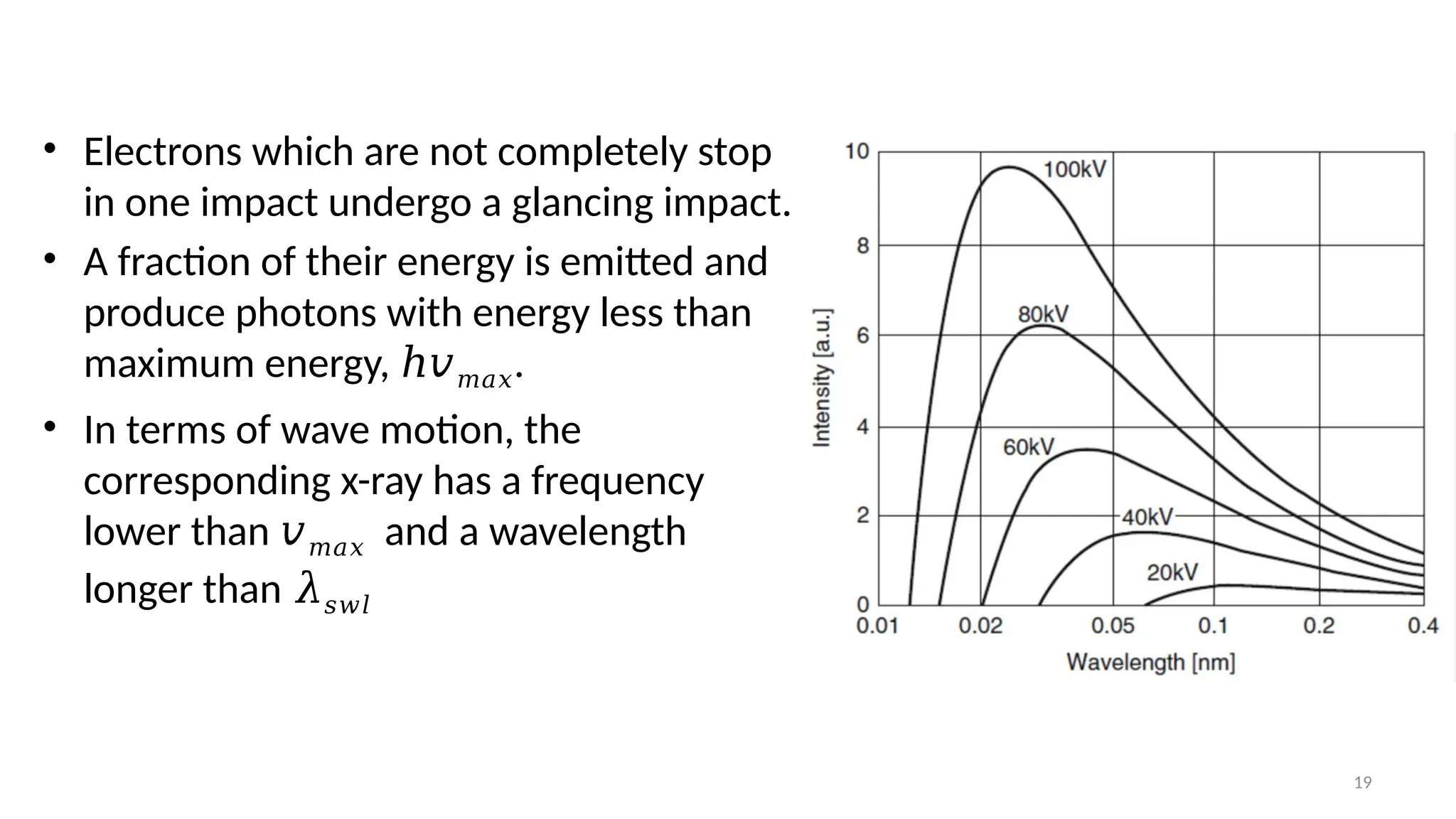
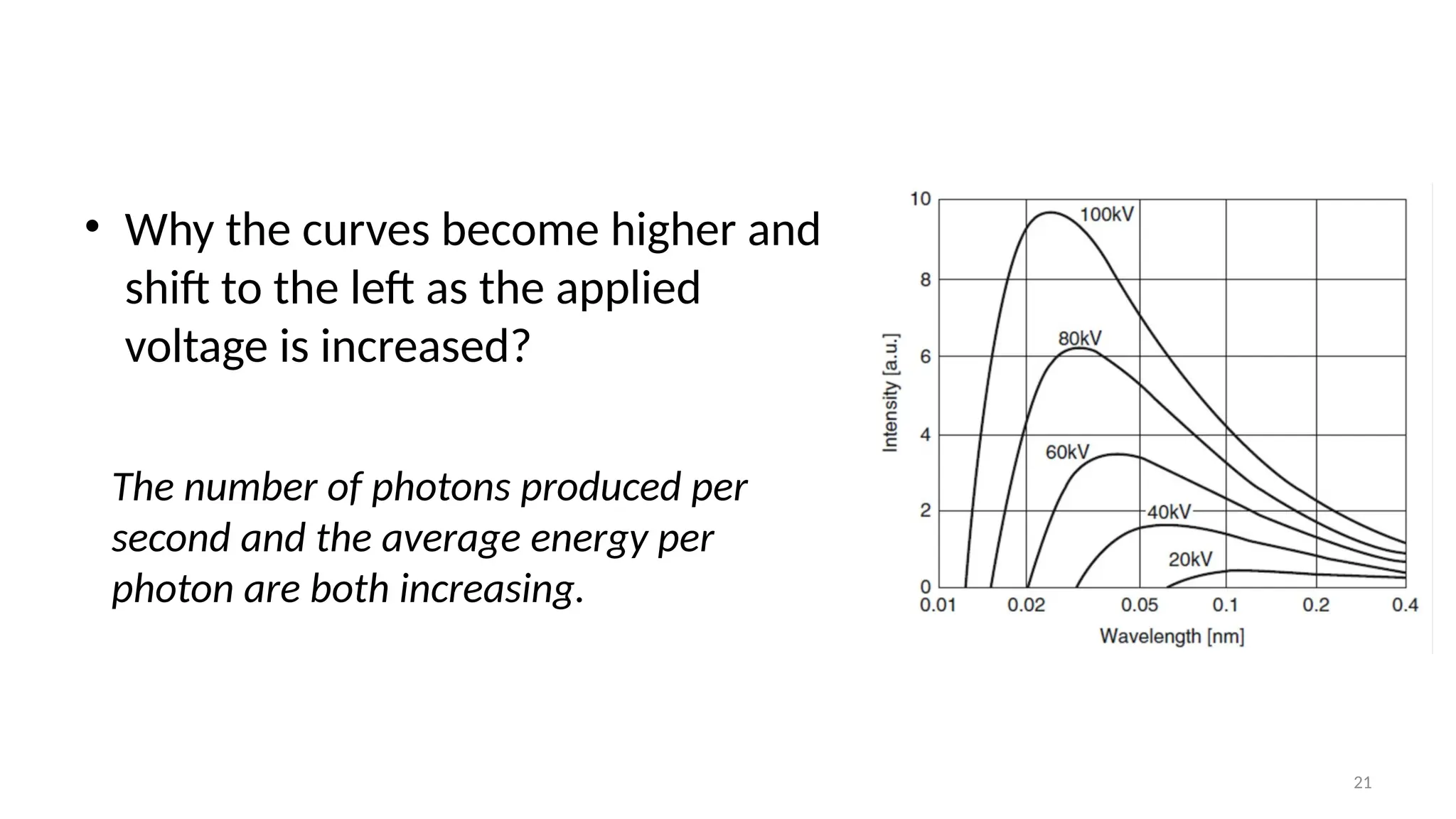
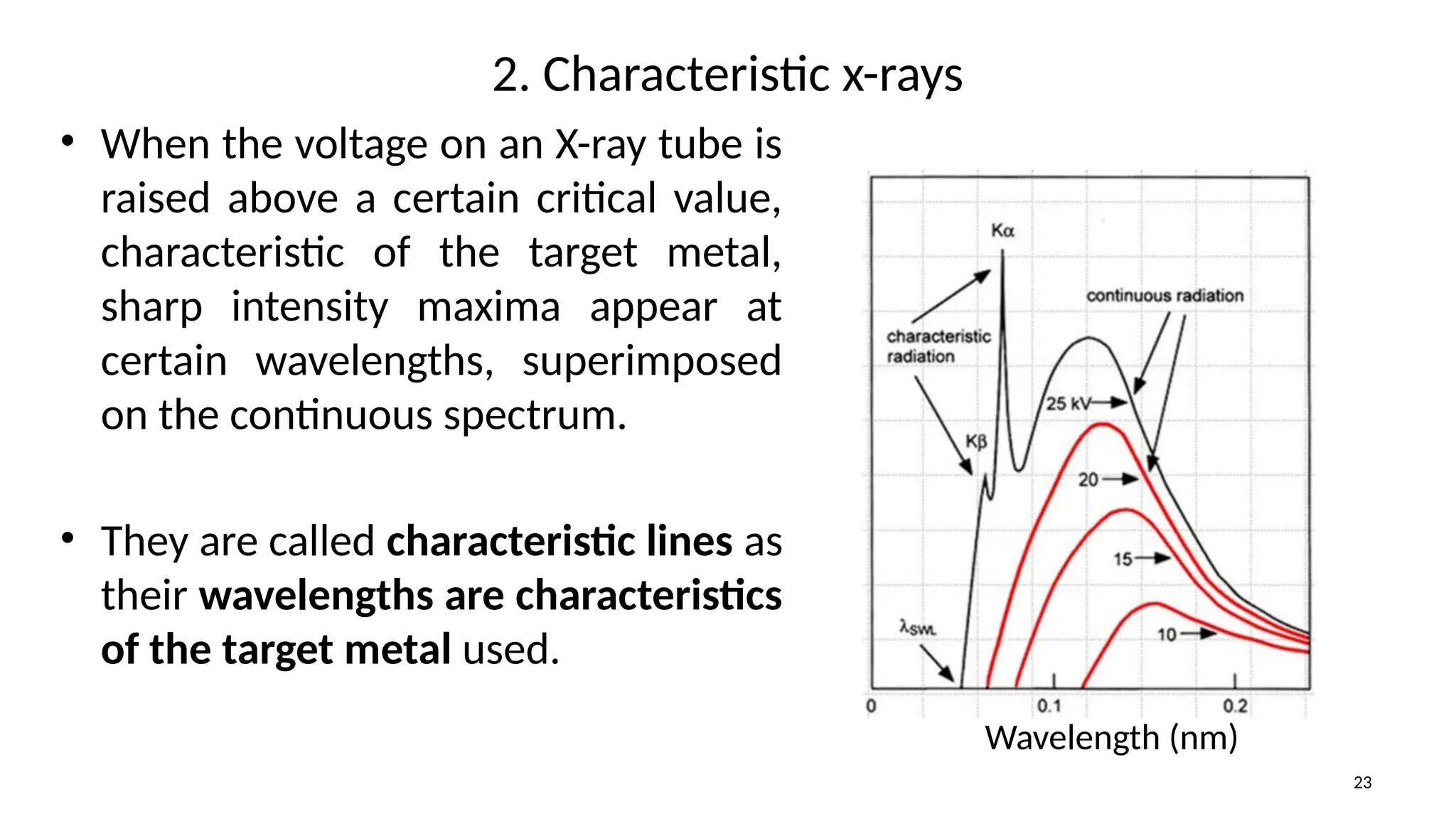
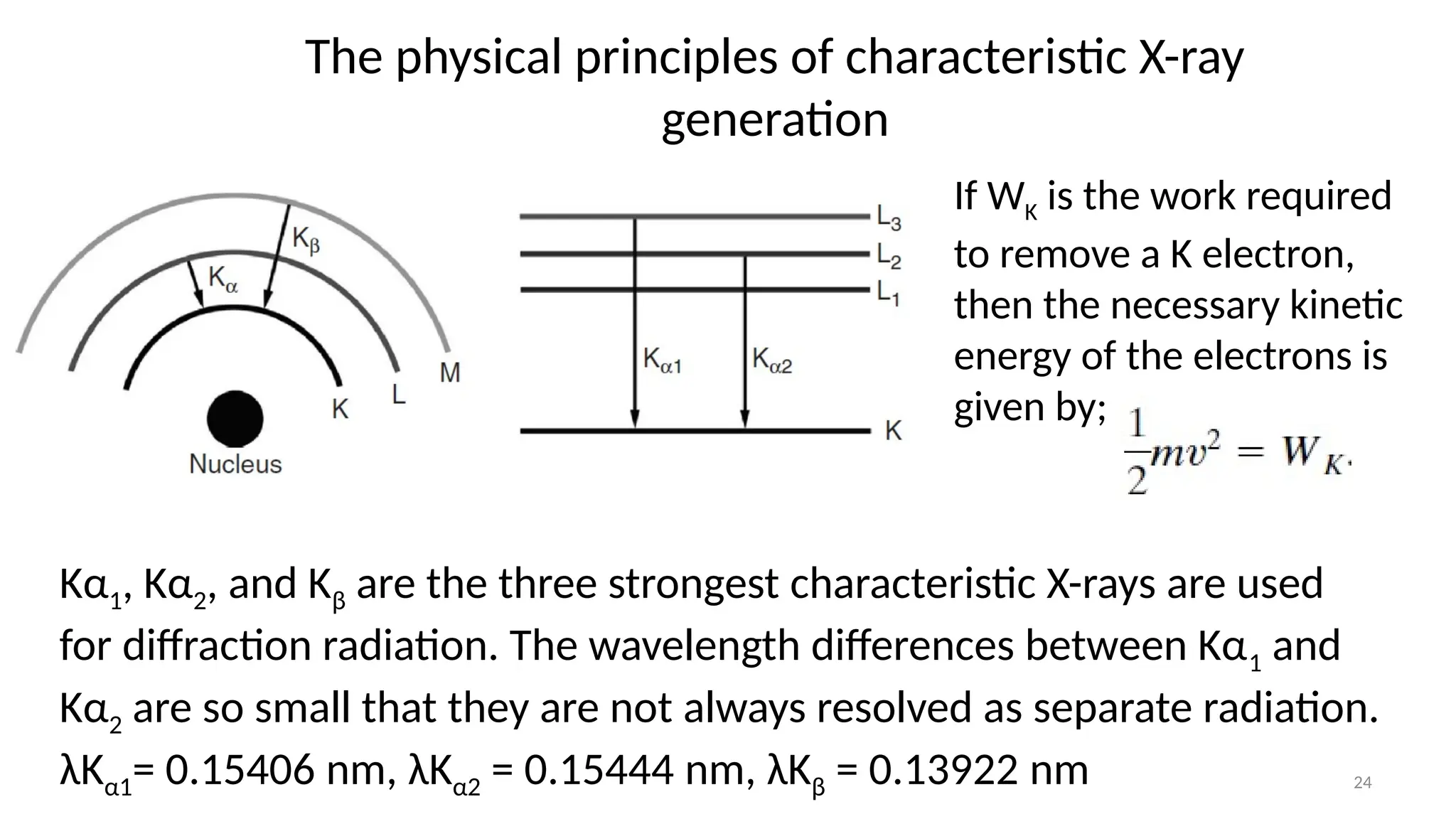
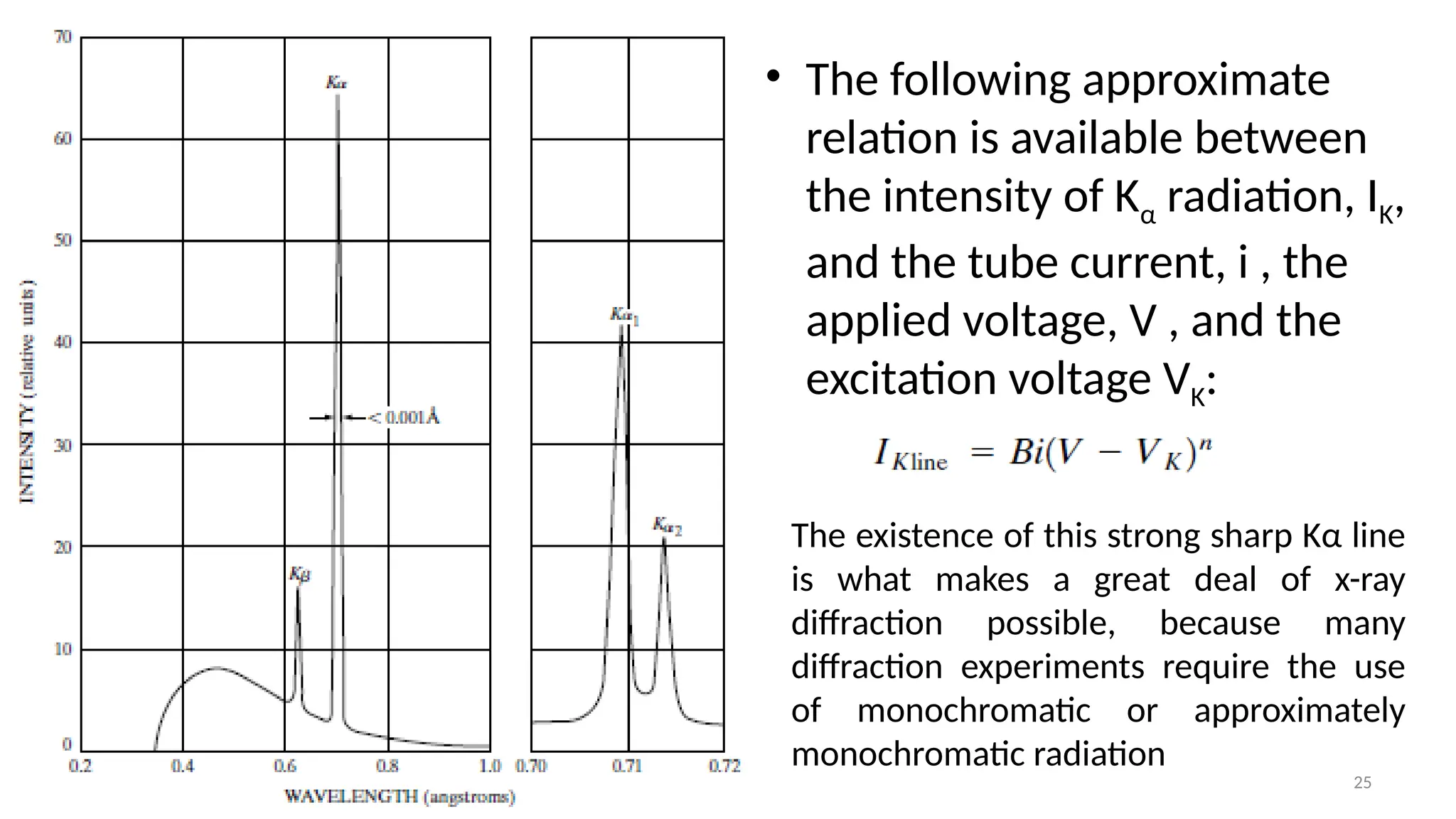
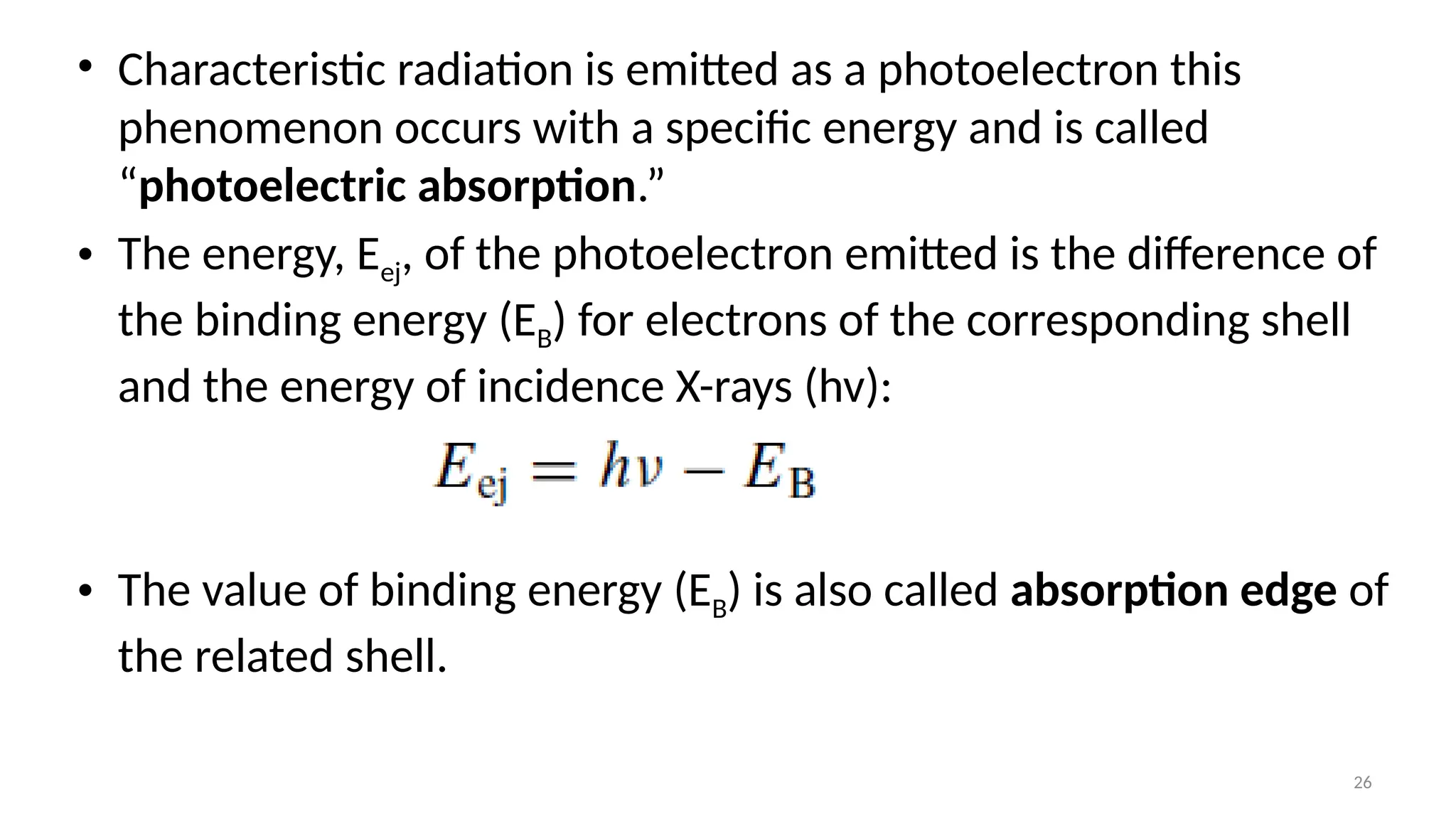
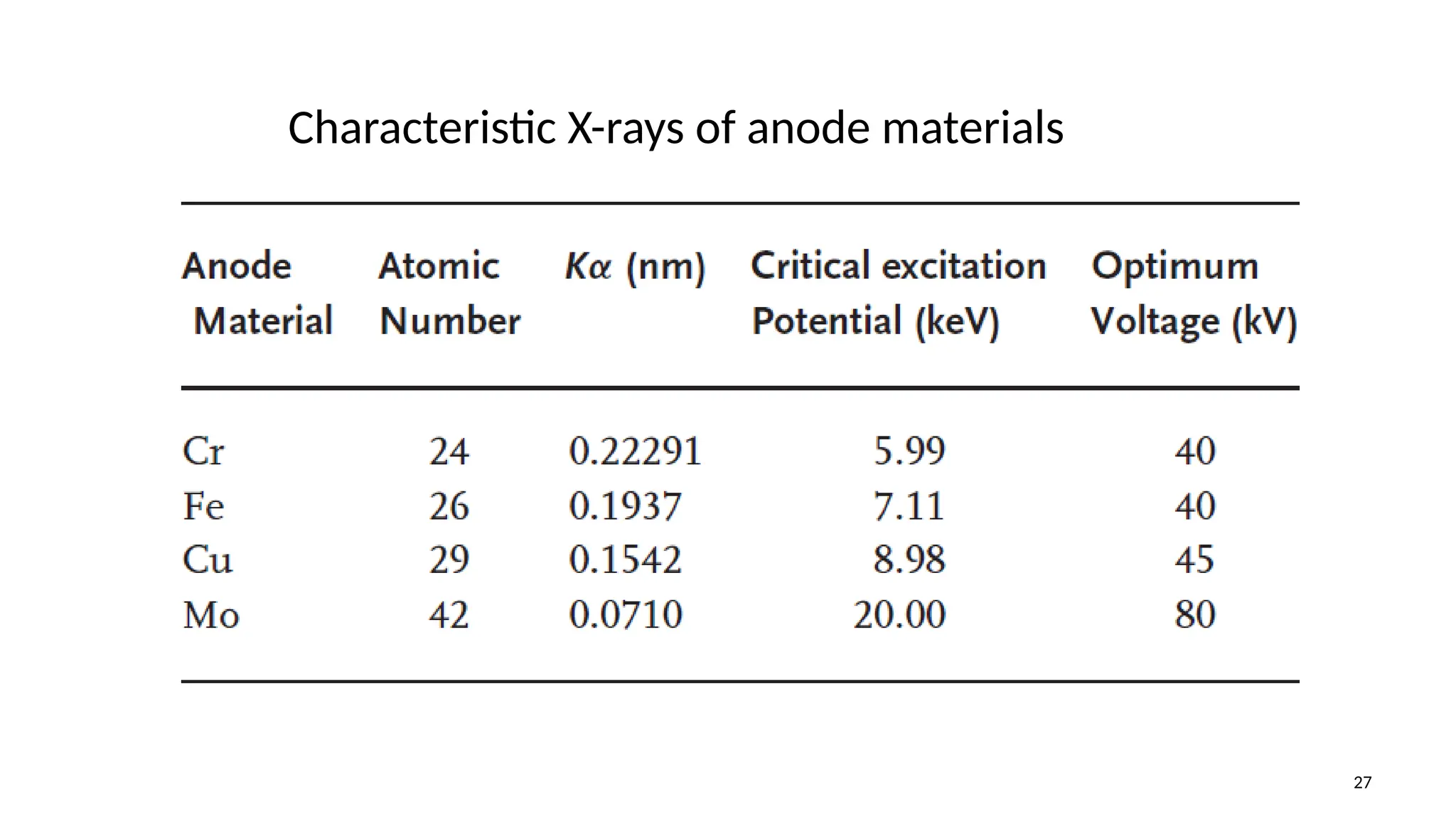
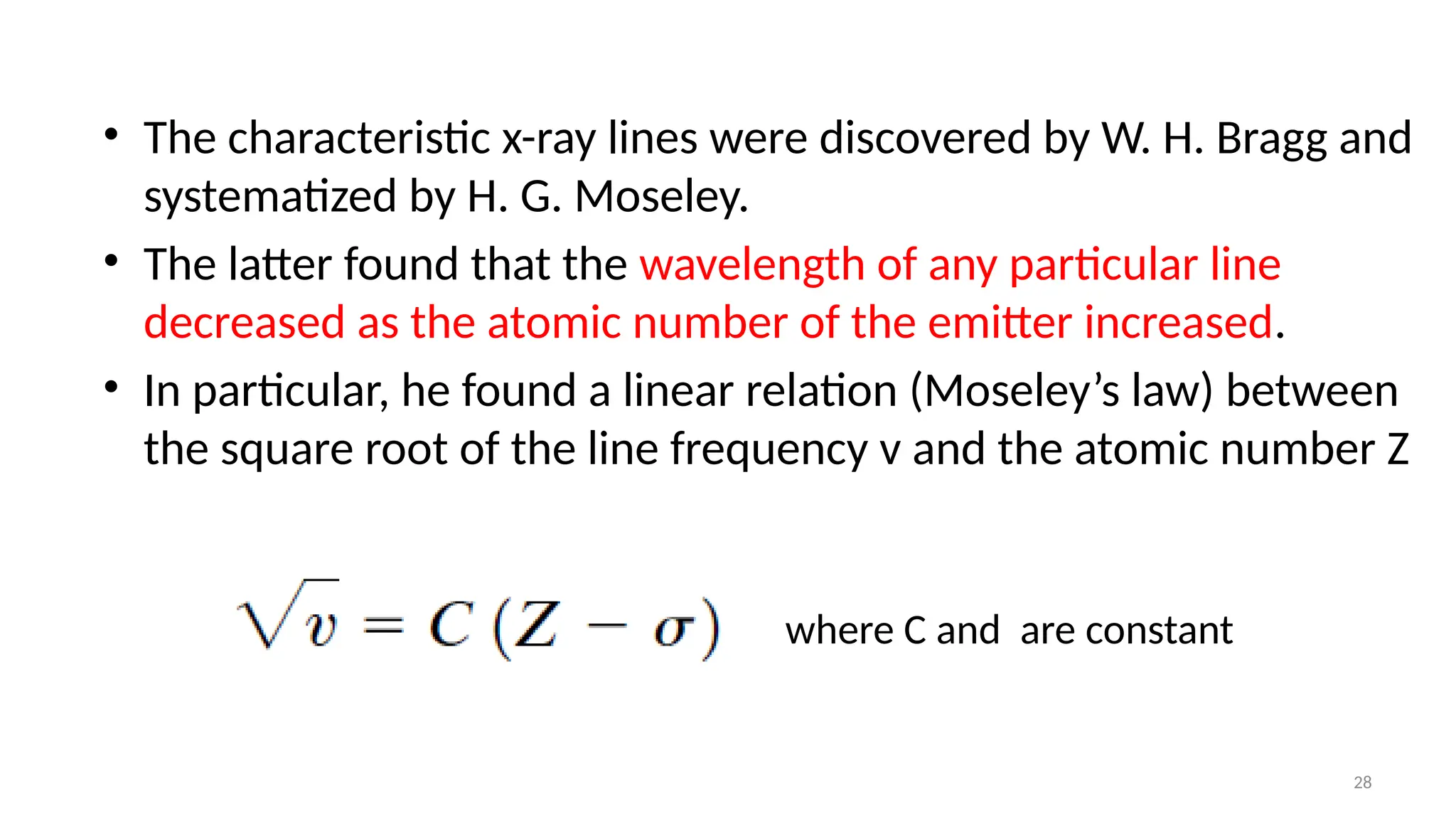
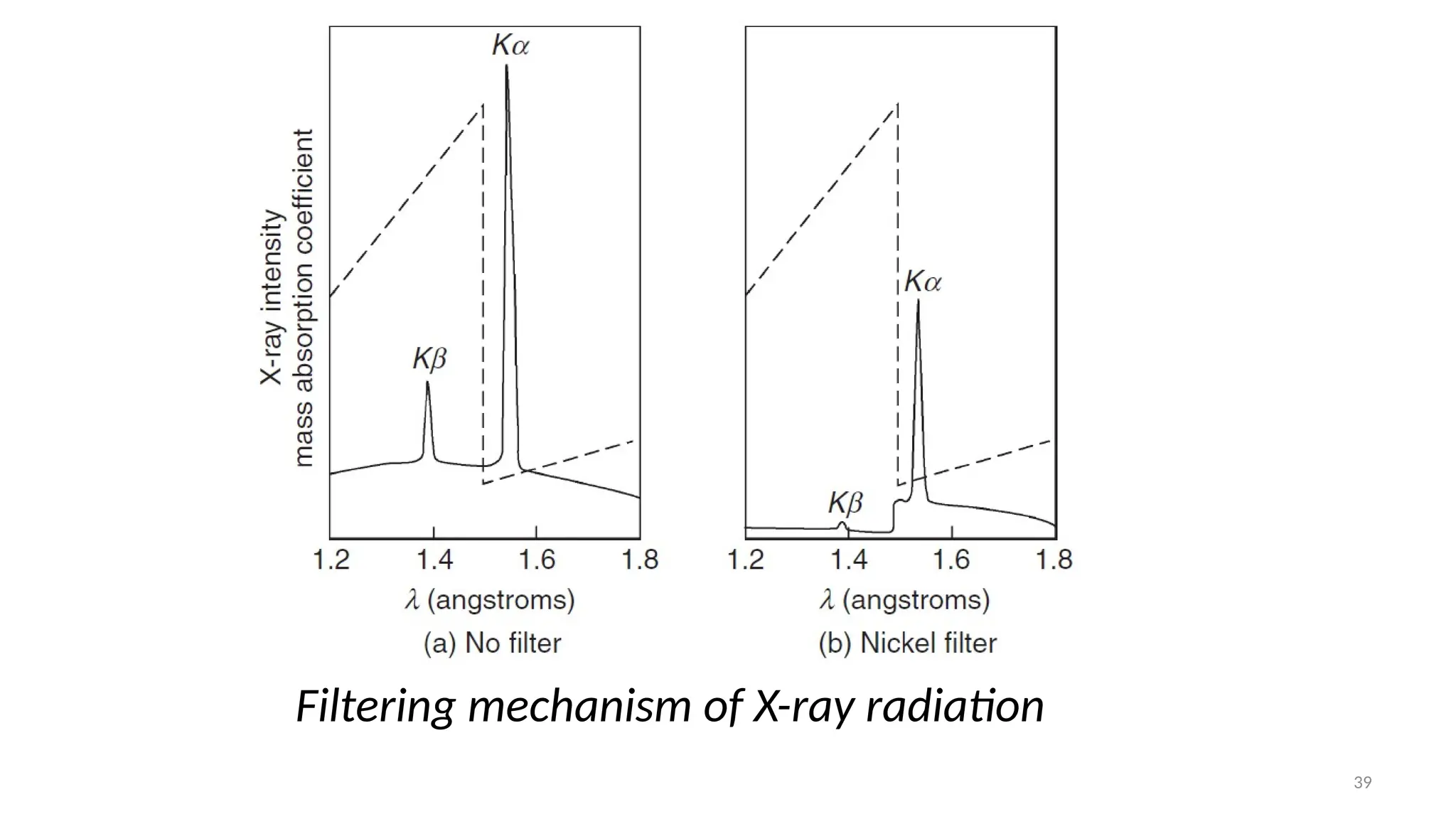
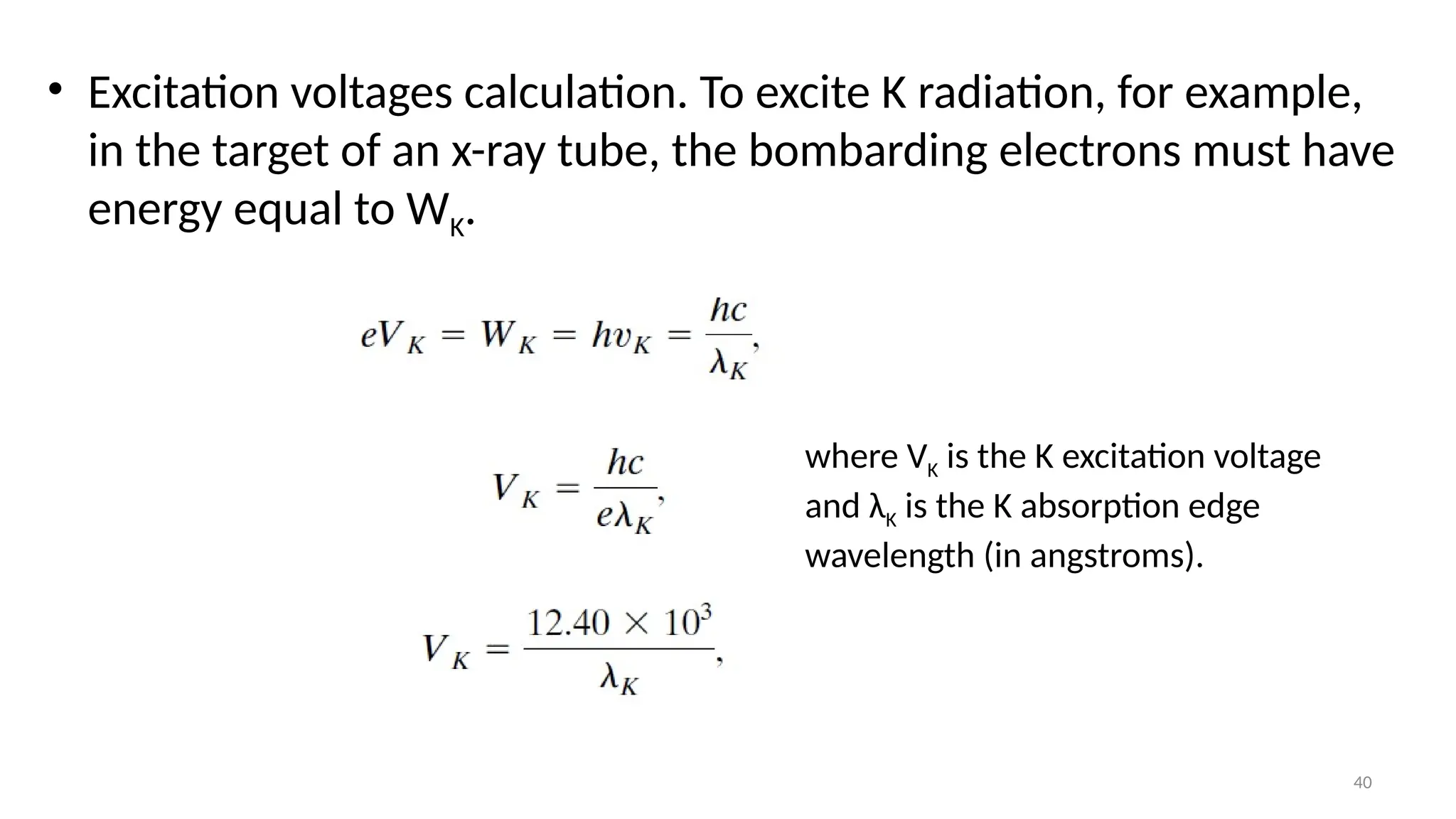
The document provides a comprehensive overview of the characterization of materials using X-rays, including their history, generation, properties, and absorption mechanisms. It details types of X-rays such as continuous and characteristic X-rays, their production processes, and safety considerations for operating X-ray equipment. Additionally, it discusses the importance of filters and detection methods for X-ray applications in scientific research and industry.