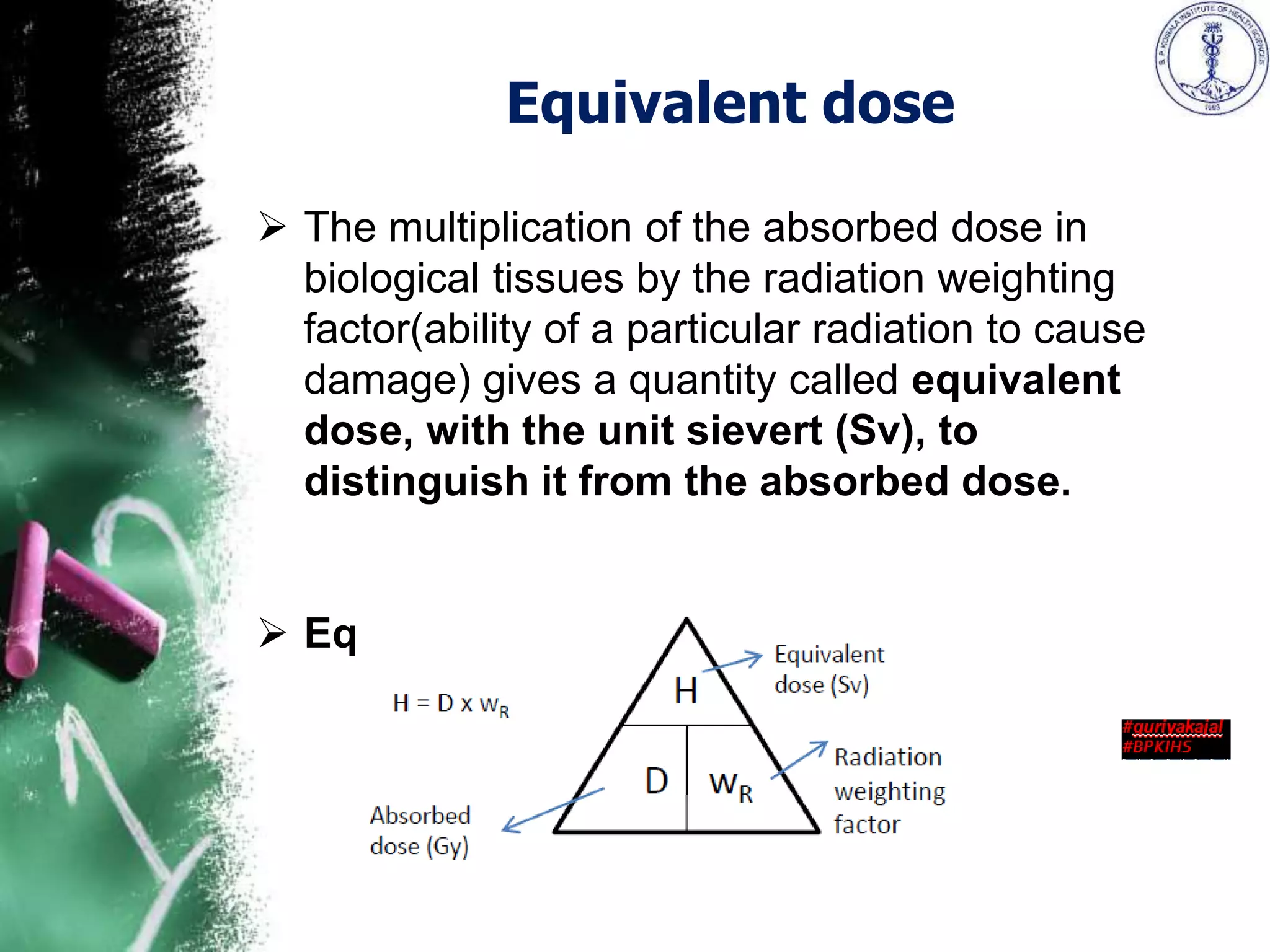
The document presents an overview of radiation, focusing on ionizing and non-ionizing radiation, and the importance of radiation protection as defined by various organizations. It discusses historical discoveries in radiation, the measurement of radiation and its biological effects, as well as the regulations and practices concerning radiation safety for individuals and patients. Key radiation units and dosimetry methods, including dosimeters used in personnel monitoring, are also outlined, emphasizing the ongoing need for safety measures and exposure limits.