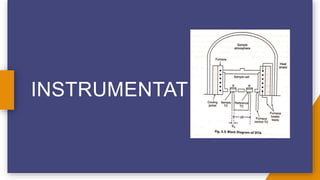
Differential thermal analysis (DTA) measures the difference in temperature between a sample and an inert reference material as they are heated at the same rate. DTA can detect phase transitions like melting or chemical reactions in a sample by observing temperature differences - endothermic events cause the sample temperature to lag behind the reference, while exothermic events cause it to rise faster, appearing as peaks on the DTA curve. The instrument consists of a furnace containing the sample and reference along with thermocouples to measure their temperatures, allowing qualitative and quantitative analysis of thermal events in materials.