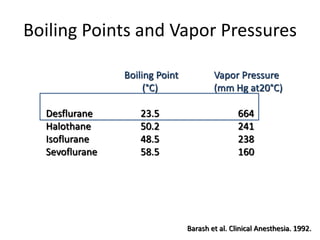
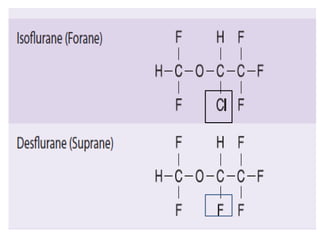
Desflurane and xenon are inhalational anesthetic gases. Desflurane was introduced in 1992 and has a rapid onset and offset of action due to its low solubility. It allows tight control of anesthetic levels but can cause increases in heart rate and blood pressure during induction. Xenon was first shown to produce anesthesia in 1951. It has analgesic properties, cardiovascular stability, and rapid induction and emergence times due to its low blood-gas partition coefficient. However, xenon is very expensive to use and its high density requires increased breathing resistance. Both agents provide specific advantages for anesthesia, but desflurane is more commonly used due to its lower cost.





![Desflurane Vaporizer : Tec 6
• electrically powered, heated, pressurized
• Output concentration: 0% to 18% (separate
safety feature for
concentrations > 12%)
• Fresh gas flow range: 0.2 to 10 L/min
• Accuracy: 0.5% absolute or 15% relative,
whichever is greater (similar to other
Tec-type vaporizers)
Weiskopf RB et al. Br J Anaesth. 1994;72:474-479; Yasuda N
et al. Anesth Analg. 1991;72:316-324; Tec 6 Vaporizer
[product brochure]. Liberty Corner, NJ: Ohmeda
Pharmaceutical Products Division Inc; 1995.](https://image.slidesharecdn.com/desandxe1-copy-200831125450/85/DESFLURANE-AND-XENON-7-320.jpg)