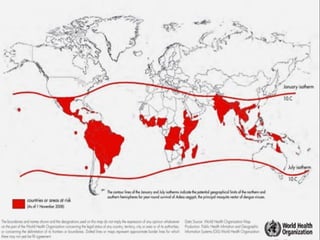
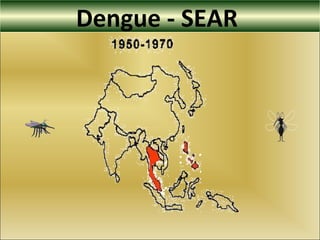
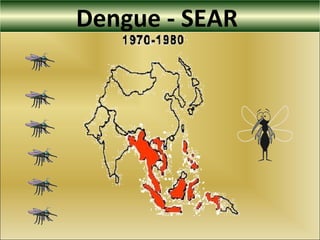
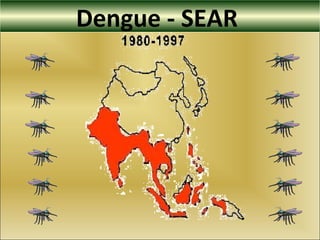
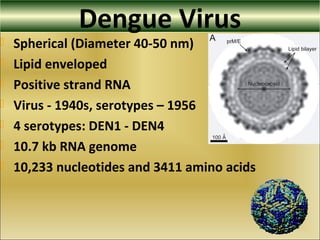
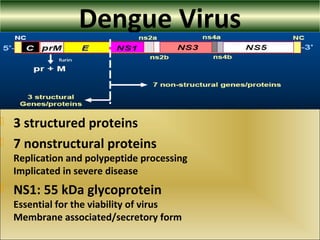
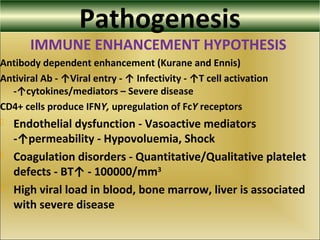
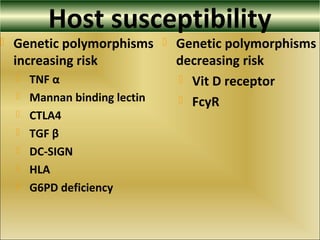
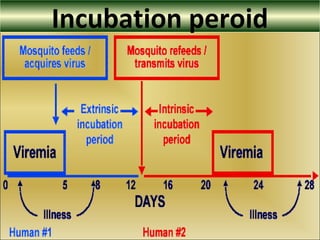
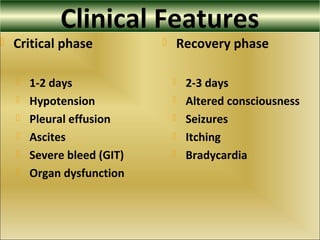
This document provides an overview of dengue virus, the disease it causes, epidemiology, pathogenesis, clinical presentation, diagnosis and prevention. It discusses the four serotypes of dengue virus and its mosquito vectors. It describes how the virus is transmitted between humans and mosquitoes. Clinical features of both dengue fever and dengue hemorrhagic fever are outlined. The document reviews various laboratory diagnostic methods including antigen detection, antibody detection, molecular detection and virus isolation. Prevention and management of dengue focuses on mosquito control and supportive care.