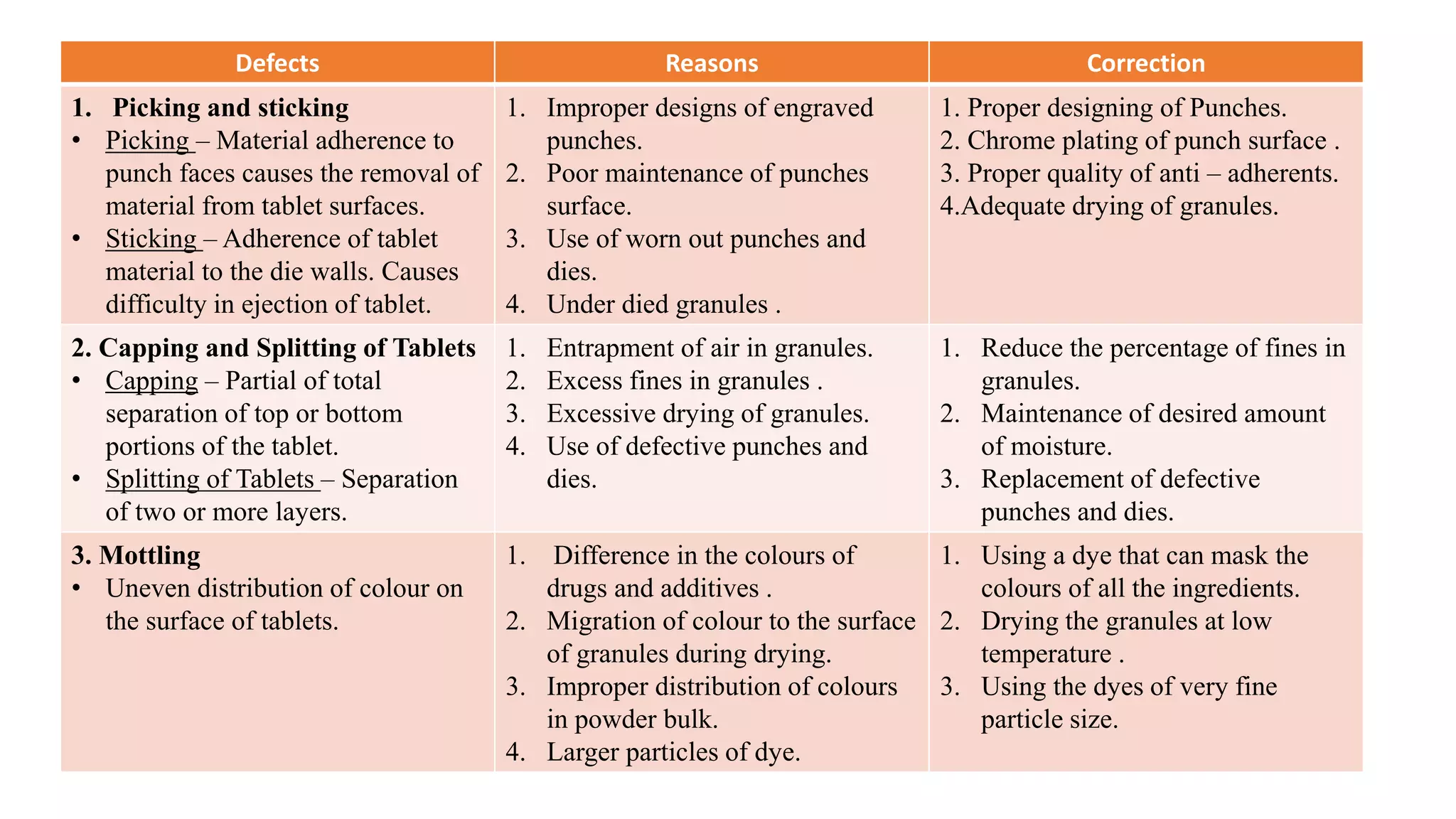
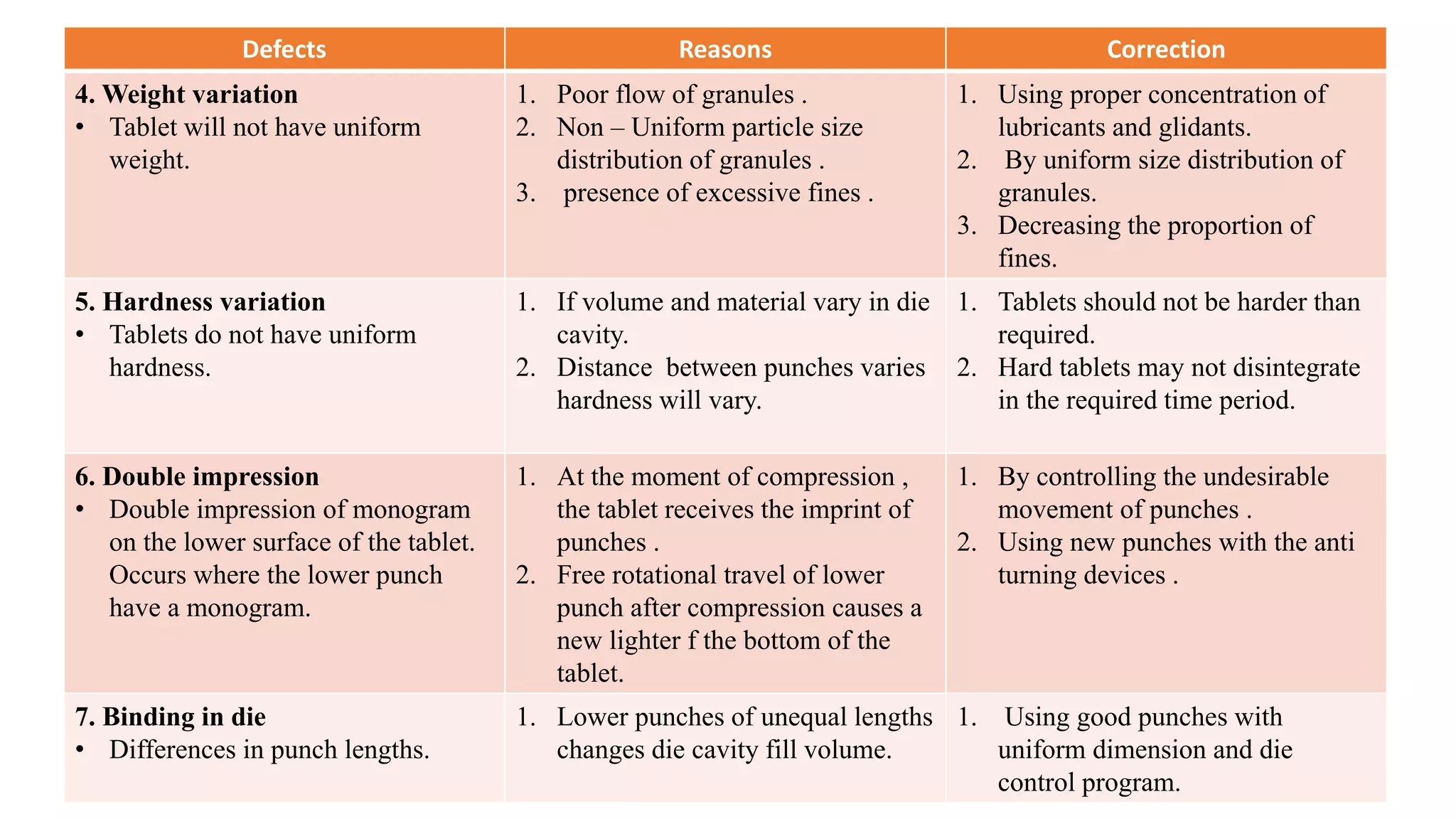
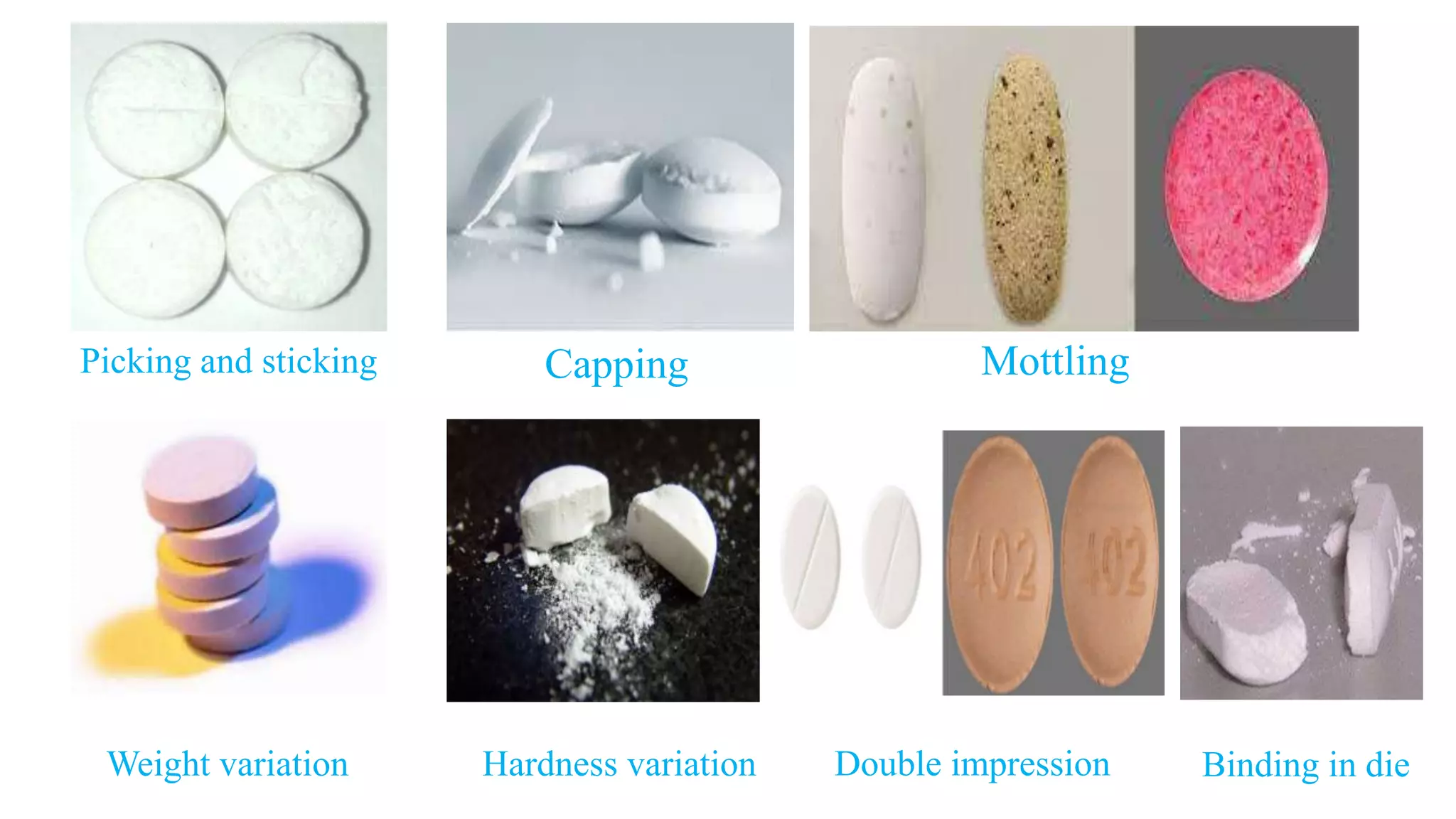
The document discusses common manufacturing defects in tablet production, including picking and sticking, capping and splitting, mottling, weight and hardness variation, double impression, and binding in die. Each defect is accompanied by its causes and suggested corrections to minimize these issues. The information is aimed at industrial pharmacists to enhance tablet manufacturing processes.