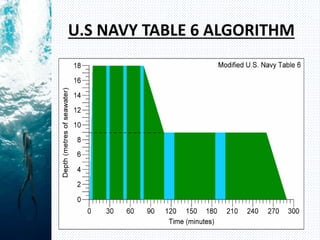
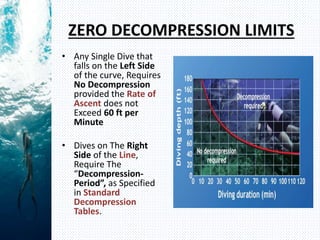
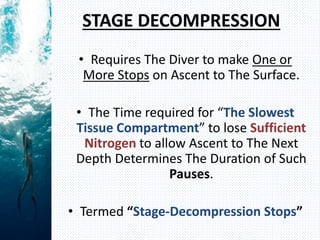
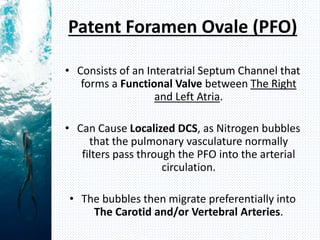
- Henry's law states that the amount of gas dissolved in a liquid is proportional to the partial pressure of that gas in contact with the liquid. During scuba diving, the increased pressure at depth causes more nitrogen to dissolve in tissues. Rapid ascent reduces pressure and causes nitrogen bubbles to form, potentially resulting in decompression sickness (DCS).
- DCS occurs when dissolved nitrogen forms bubbles in tissues during ascent. It can be prevented by following safe ascent procedures and dive tables. Treatment involves recompression in a hyperbaric chamber to resolve the bubbles.