This document discusses several key concepts in diving physiology:
1) It describes fundamental laws of physics that govern diving like Boyle's law on gas volume and pressure changes, and Dalton's law on partial pressures of gases.
2) It explains physiological effects of pressure on the body and potential issues like barotrauma, nitrogen narcosis, oxygen toxicity, and decompression sickness.
3) It provides an overview of different types of diving and factors like buoyancy, temperature changes, and characteristics of common gases like oxygen, nitrogen, and helium.





















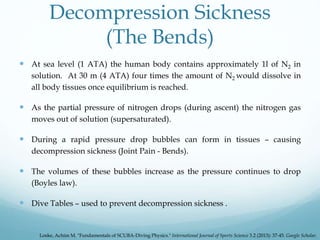







![Decompression Sickness
While an individual can do everything
correctly and still suffer from DCS, prevention
can be enhanced if:
Ascend slowly (30 ft/min [9 m/min])
Make safety stops
Use longer surface intervals
Plan the dive, dive the plan and have a backup plan
Maintain good physical fitness, nutrition, and
hydration](https://image.slidesharecdn.com/d6c55366-ea88-4468-abfe-f4bbc287a4de-150606083203-lva1-app6892/85/Elective-Presentation-Final-2-30-320.jpg)




