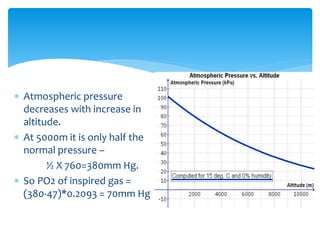
This document discusses respiratory physiology at high altitudes. It begins by classifying altitudes and explaining how atmospheric pressure decreases with increasing altitude. The body adapts to high altitudes through hyperventilation, polycythemia, and shifts in the oxygen-hemoglobin dissociation curve. Acute mountain sickness can occur if the ascent is too rapid and symptoms include headache, fatigue, and nausea. Other issues discussed include high altitude pulmonary and cerebral edema, chronic mountain sickness, oxygen toxicity, and respiratory changes during space flight and scuba diving.

![ High altitude = 1,500–3,500 metres (4,900–11,500 ft)
Very high altitude = 3,500–5,500 metres (11,500–18,000 ft)
Extreme altitude = above 5,500 metres (18,000 ft)
The death zone - altitudes above a certain point where the
amount of oxygen is insufficient to sustain human life. This
point is generally tagged as 8,000 m (26,000 ft) [less than
356 millibars of atmospheric pressure]
Classification of heights](https://image.slidesharecdn.com/respiratoryphysiologyathighaltitudes-150912061355-lva1-app6892/85/Respiratory-physiology-at-high-altitudes-2-320.jpg)