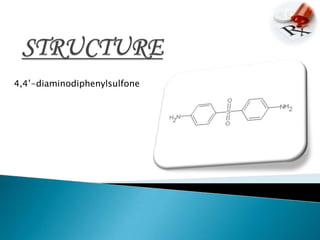
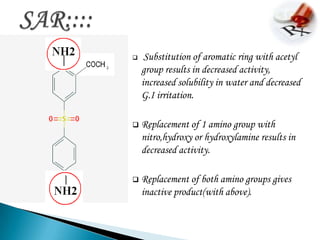
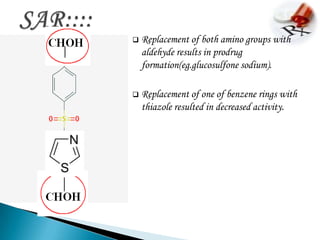
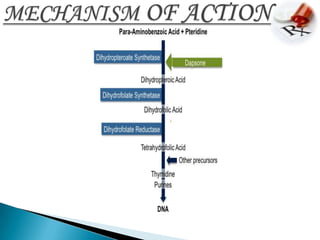
This document summarizes information about the drug dapsone, including:
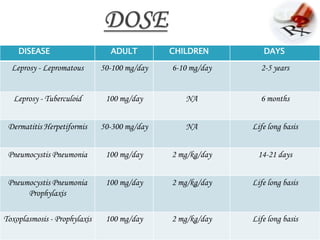
1. Dapsone was invented in the early 20th century and is commonly used to treat leprosy in combination with other drugs.
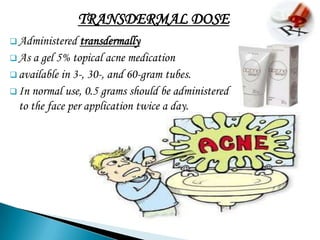
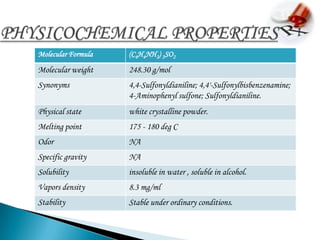
2. It is absorbed after oral administration and metabolized in the liver. Common side effects include anemia, nausea, and rashes.
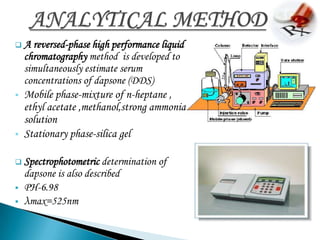
3. Analytical methods like HPLC and spectroscopy can be used to detect and quantify dapsone in samples.