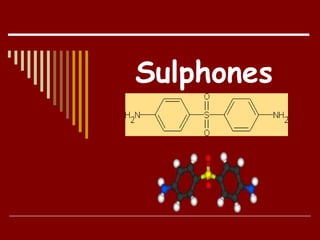
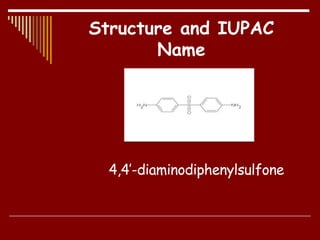
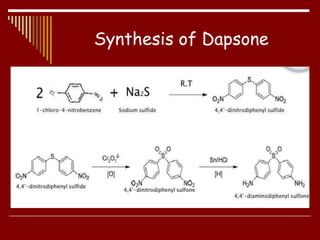
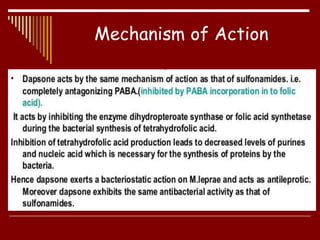
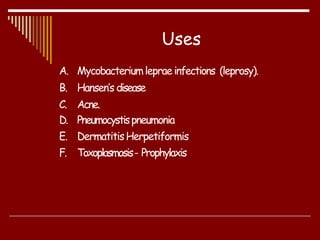
Dapsone is a medication most commonly used in combination with rifampicin and clofazimine as multidrug therapy for leprosy treatment. It was independently discovered in the 1930s-1940s and acts as an antibacterial by inhibiting the synthesis of dihydrofolic acid. Common adverse effects include mild haemolytic anaemia, gastric intolerance, and methaemoglobinaemia. Dapsone's mechanism of action involves inhibiting the enzyme dihydropteroate synthetase to block bacterial folic acid synthesis, and it may also have an anti-inflammatory effect through an unknown mechanism.