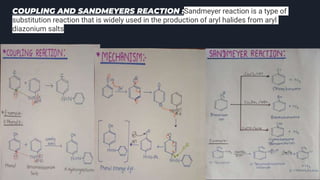
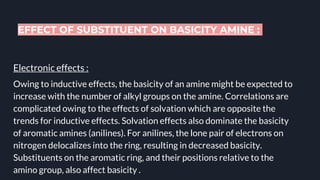
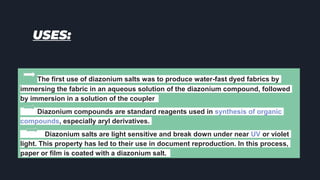
This document provides an overview of aromatic amines. It begins with an introduction noting that aromatic amines are industrially important compounds used to produce dyes, rubbers, and drugs. It then discusses the classification, characteristics, and reactions of aromatic amines. Key points covered include that aromatic amines are weaker bases than aliphatic amines due to delocalization of the nitrogen lone pair onto the aromatic ring. The document also discusses substituent effects on basicity and the uses of aryl diazonium salts in organic synthesis and light-sensitive applications. Presentations were given on specific topics like nitrosation reactions, coupling reactions, and the Hinsberg test.