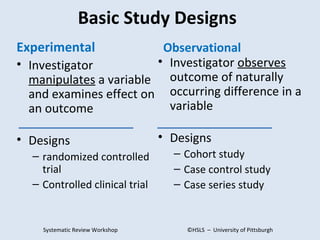
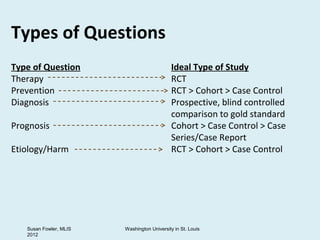
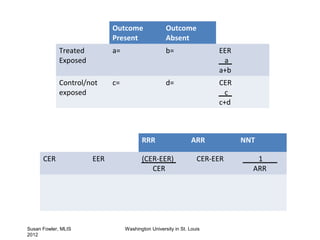
The document provides an overview of critical appraisal of medical research. It discusses study designs such as randomized controlled trials, observational studies, systematic reviews and meta-analyses. It also covers topics like developing PICO(T) questions to frame clinical questions, different types of study questions, evaluating studies using the FRISBE mnemonic, calculating measures of clinical importance, and resources for evidence-based practice.