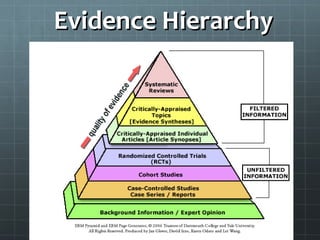
This document outlines the steps of the evidence-based medicine process which includes assessing the patient, asking a question, acquiring resources, appraising the evidence, and applying to the patient. It discusses how to formulate a well-built clinical question using PICO(TT) and describes the different types of study designs and evidence hierarchies. Guidelines are provided for evaluating the quality of individual studies, such as using the FRISBE checklist, and for critically appraising systematic reviews and meta-analyses.