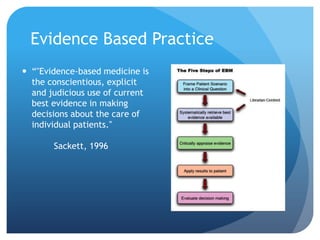
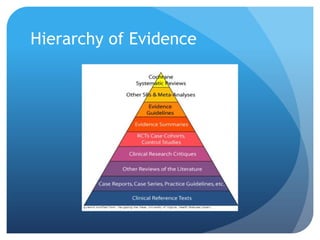
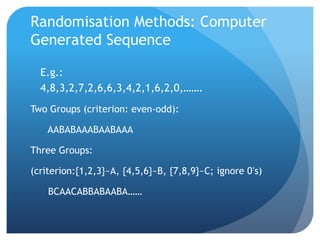
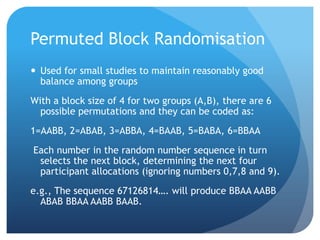
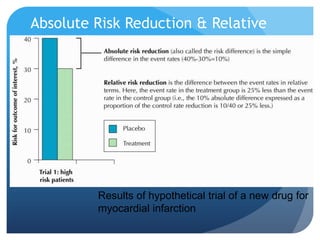
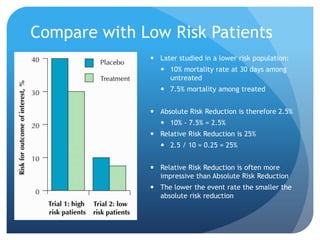
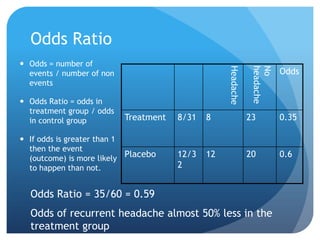
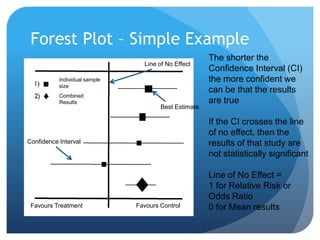
This document provides an introduction to critical appraisal. It defines critical appraisal as systematically weighing the quality and relevance of research to inform decision making. The document outlines different types of research studies including systematic reviews, randomized controlled trials, cohort studies, and case-control studies. It discusses how to critically appraise studies by assessing their validity, results, and relevance. Key aspects of appraising randomized controlled trials are described such as randomization, blinding, accounting for all participants, and interpreting results including p-values and confidence intervals. The goal is to help readers gain skills to critically evaluate research.