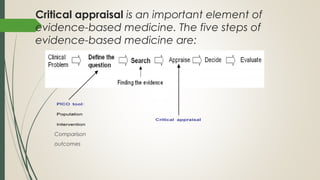
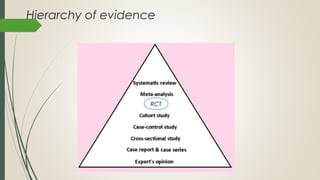
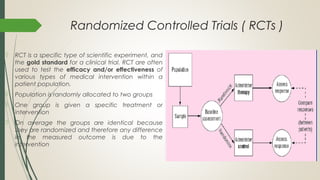
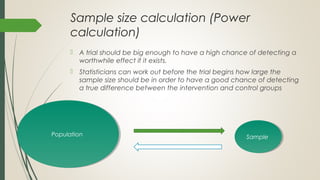
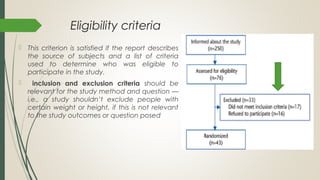
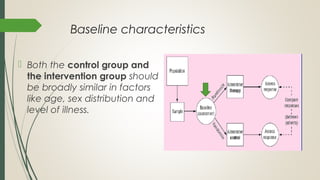
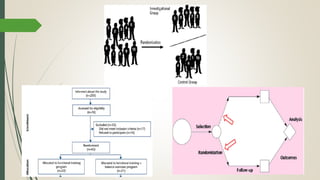
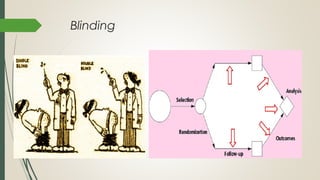
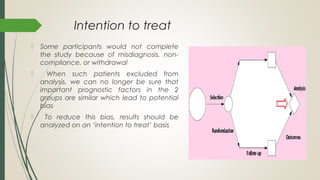
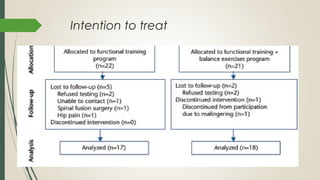
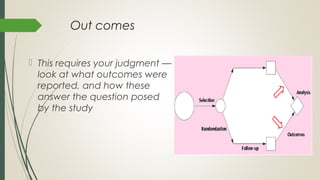
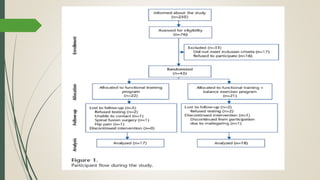
This document provides an overview of critical appraisal of randomized controlled trials (RCTs). It defines critical appraisal as carefully examining research to assess its trustworthiness and relevance. RCTs are described as the gold standard for clinical trials, where participants are randomly allocated to groups that receive either a treatment or a control. Key factors to examine in appraising an RCT are described, including sample size, eligibility criteria, baseline characteristics, randomization, blinding, follow-up of participants, data collection, presentation of results, and applicability to local populations. Advantages of critical appraisal and RCTs include providing a systematic way to assess research validity and improving practice, while disadvantages include taking time and not always finding clear answers.