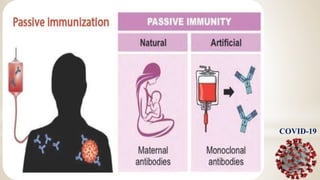
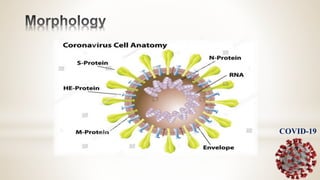
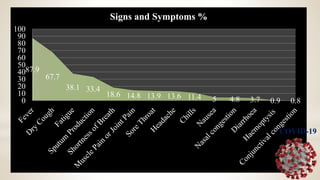
This document discusses immunization and passive immunization, with a focus on COVID-19. It defines immunization as making a person immune or resistant to disease by vaccine administration. Passive immunization involves administering preformed antibodies. The document classifies coronaviruses and describes SARS-CoV-2, the virus that causes COVID-19, as closely related to SARS. It lists COVID-19 symptoms and discusses diagnosis, convalescent plasma therapy trials to treat patients, and the potential use of antibodies from recovered patients to provide immunity to others.







![Coronaviruses are classified into three groups,
The HCoVs 229E and HCoV NL63 are group 1
coronaviruses[6]
HCoV OC43, HCoV HKU-1 and SARS
coronaviruses are classified in group 2[6]
GROUP 3 Is avian strains Causing infection in
chickens
COVID-19](https://image.slidesharecdn.com/passiveimmunizationcoronavirus-220924171147-15c51171/85/passive-immunization-corona-virus-pptx-9-320.jpg)




![The NIBD head, Dr Tahir Shamshi, who had proposed
the technique to the government authorities for the
treatment of COVID-19 last month, told [1]
The Express Tribune that the process to extract plasma
from recovered coronavirus patients[2]
COVID-19](https://image.slidesharecdn.com/passiveimmunizationcoronavirus-220924171147-15c51171/85/passive-immunization-corona-virus-pptx-16-320.jpg)

![Caregivers are resorting to using century-old
Convalescent plasma therapy — siphoning blood
from survivors and reinfusing it into the sick[3]
COVID-19](https://image.slidesharecdn.com/passiveimmunizationcoronavirus-220924171147-15c51171/85/passive-immunization-corona-virus-pptx-18-320.jpg)



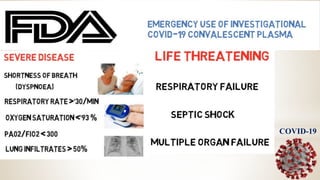
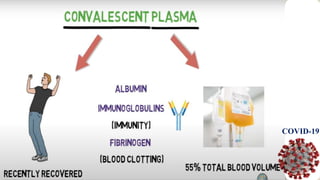



![Recovered patient can donate his blood plasma after
every two weeks which could save the live of at least
one patient.[1]
COVID-19](https://image.slidesharecdn.com/passiveimmunizationcoronavirus-220924171147-15c51171/85/passive-immunization-corona-virus-pptx-27-320.jpg)


