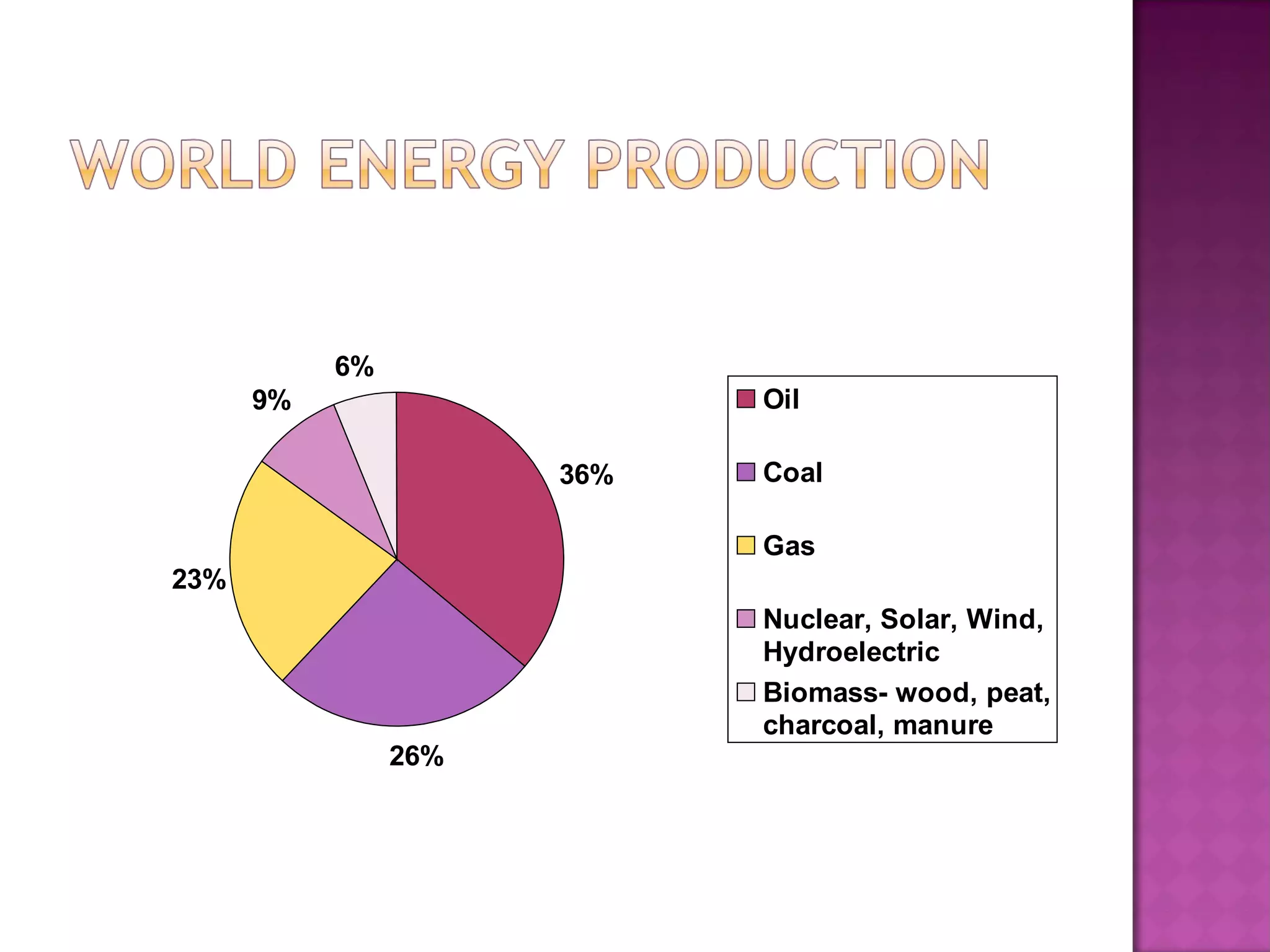
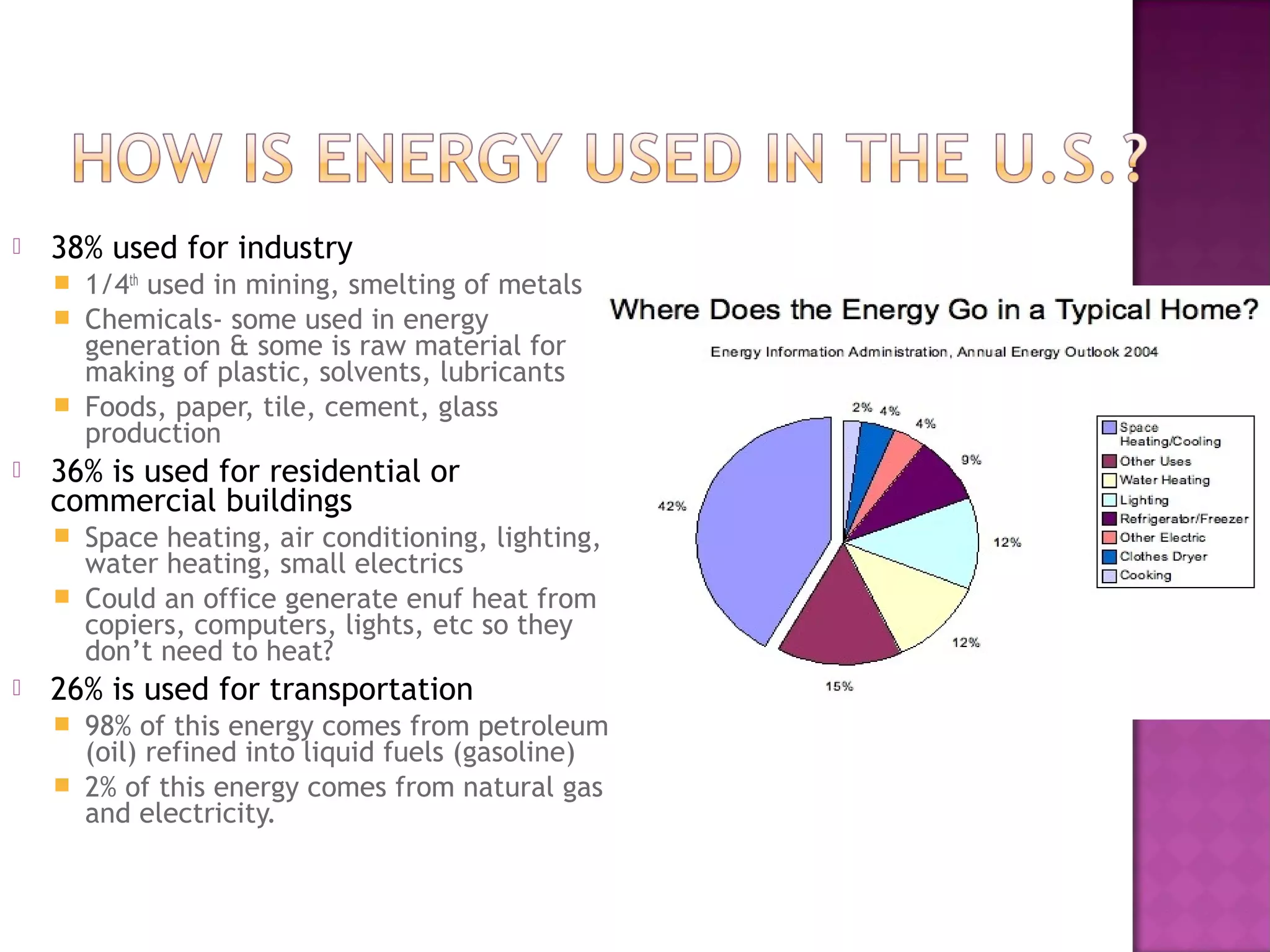
This document discusses conventional energy sources such as fossil fuels including oil, natural gas, and coal. It provides details on:
- Where these energy sources come from and how they are formed over long periods of time
- The extraction and processing methods used to produce usable fuels from these resources
- How these conventional fuels are used today to power transportation, generate electricity, heat homes and more, but also have disadvantages like greenhouse gas emissions and finite supply.



























![Natural gas is a naturally occurring hydrocarbon gas mixture consisting
primarily of methane, but commonly includes varying amounts of other
higher alkanes and even a lesser percentage of carbon dioxide, nitrogen
, and hydrogen sulfide.[1]
Natural gas is an energy source often used for
heating, cooking, and electricity generation. It is also used as fuel for
vehicles and as a chemical feedstock in the manufacture of plastics and
other commercially important organic chemicals.
Natural gas is found in deep underground natural rock formations or
associated with other hydrocarbon reservoirs in coal beds and as
methane clathrates. Petroleum is also another resource found in
proximity to and with natural gas. Most natural gas was created over
time by two mechanisms: biogenic and thermogenic. Biogenic gas is
created by methanogenic organisms in marshes, bogs, landfills, and
shallow sediments. Deeper in the earth, at greater temperature and
pressure, thermogenic gas is created from buried organic material.[2][3]](https://image.slidesharecdn.com/conventionalenergysources-161219140641/75/Conventional-energy-sources-30-2048.jpg)












