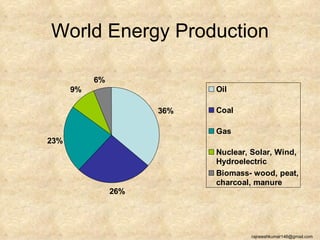
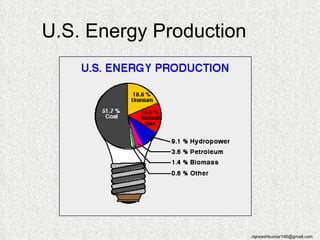
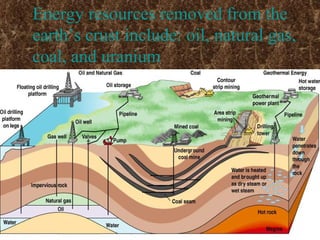
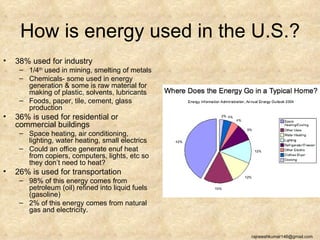
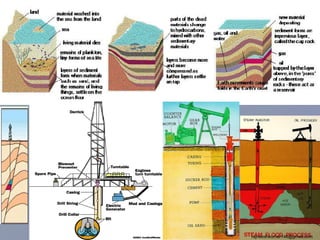
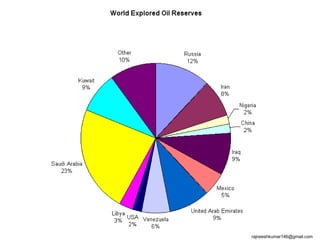
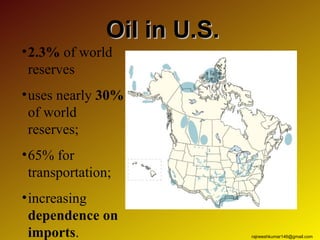
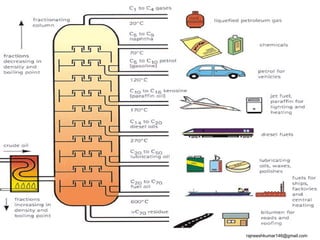
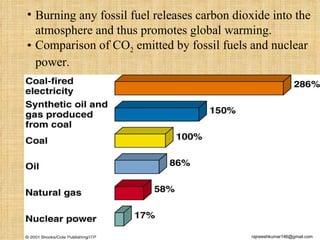
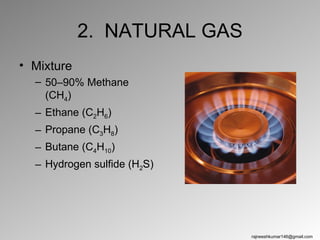
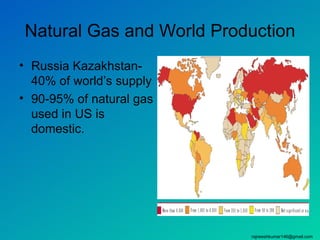
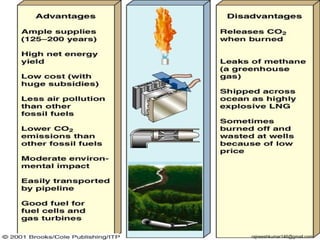
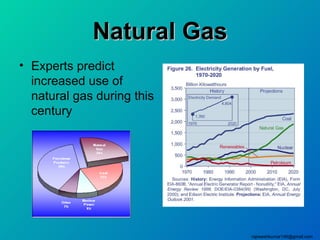
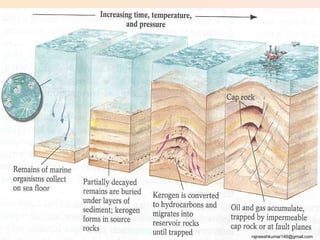
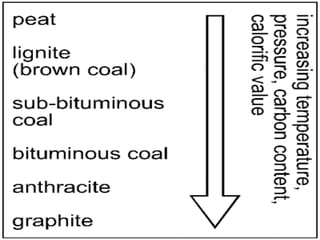
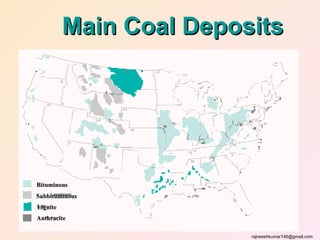
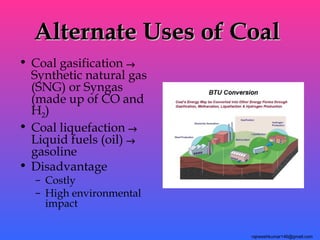
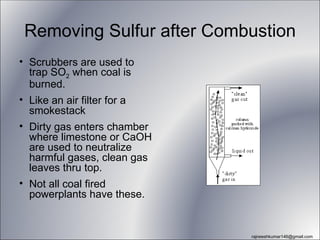
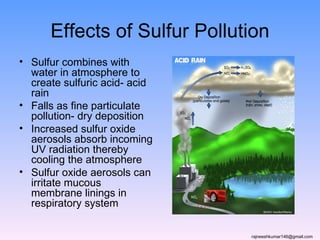
The three major conventional energy sources are petroleum, natural gas, and coal. Together they provide about 85% of the country's energy and almost two-thirds of electrical power. While widely used for decades, efforts are made to reduce their environmental impact. These non-renewable resources will be depleted if consumption continues at projected rates.