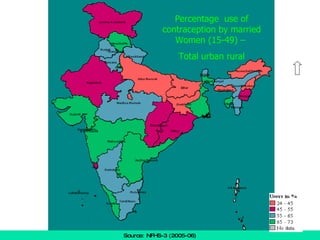
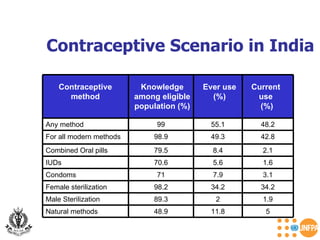
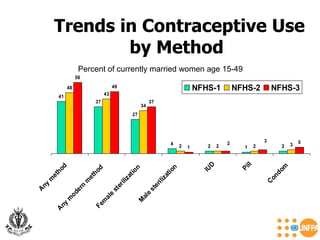
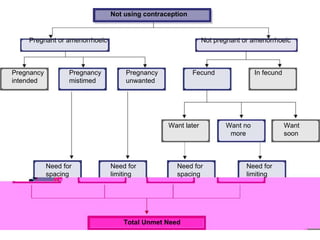
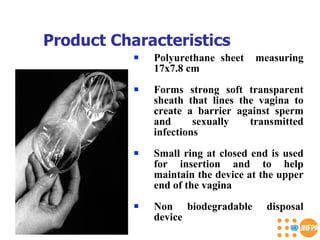
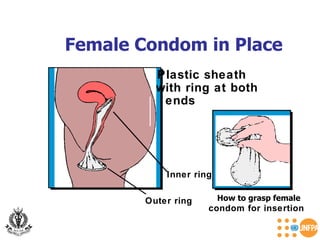
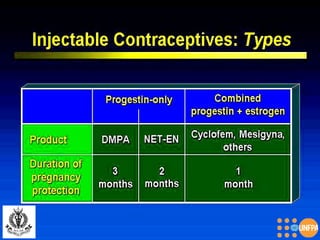
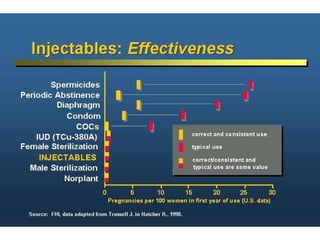
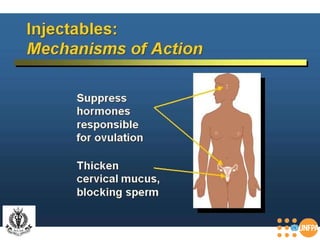
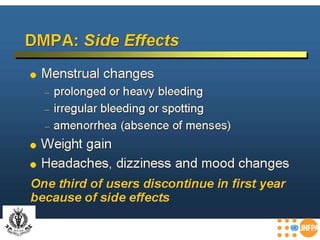
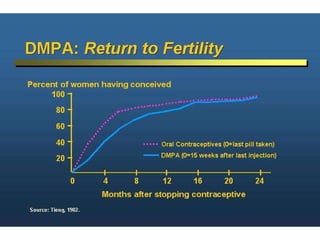
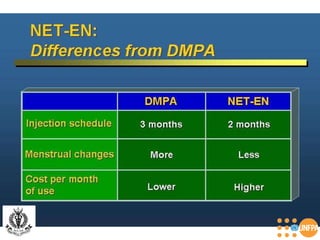
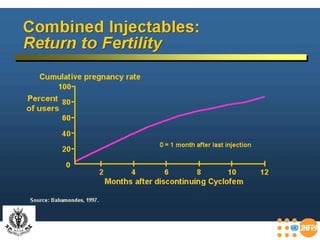
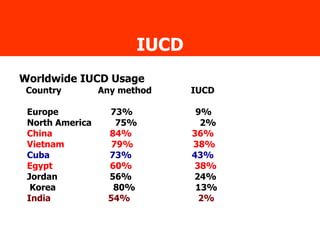
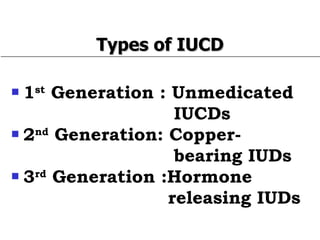
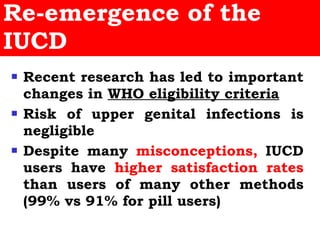
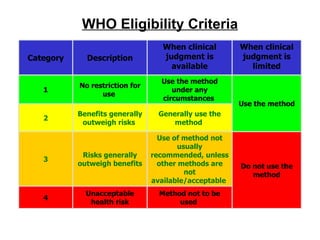
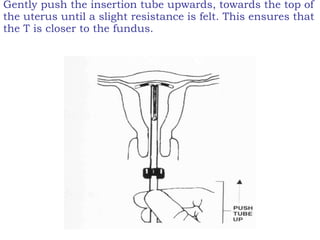
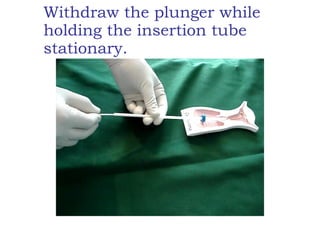
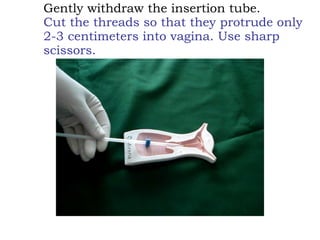
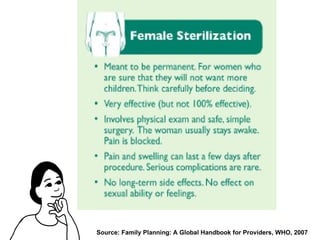
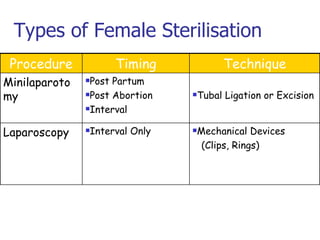
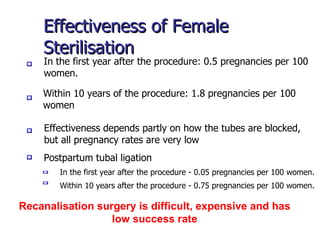
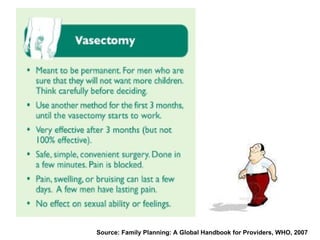
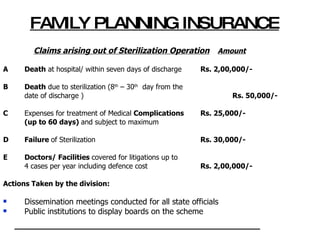
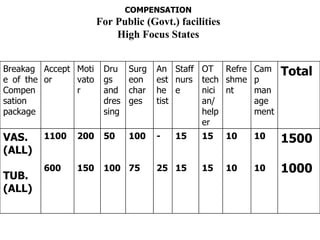
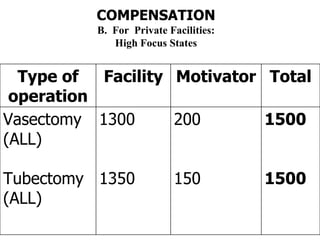
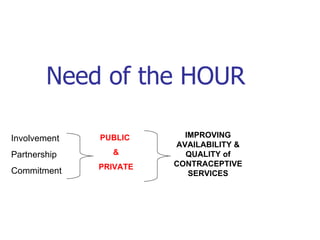
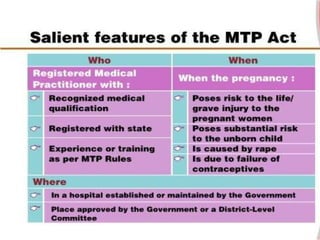
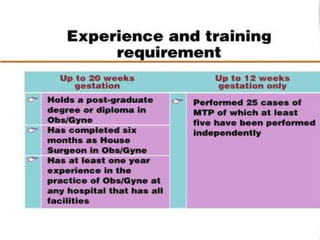
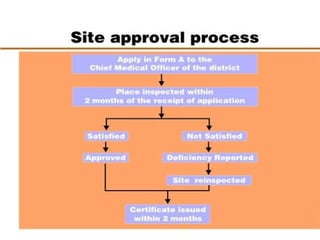
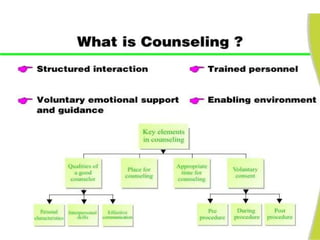
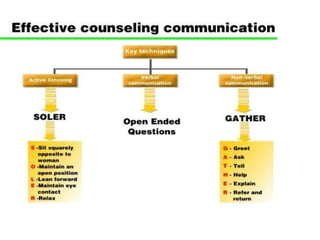
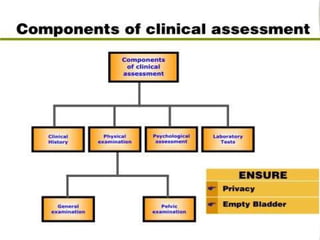
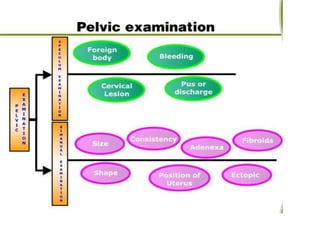
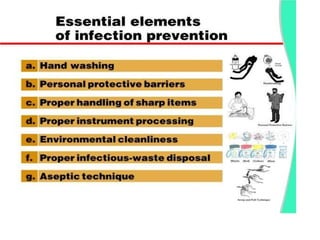
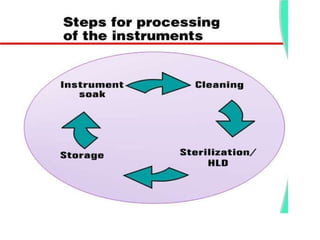
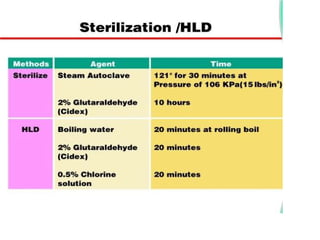
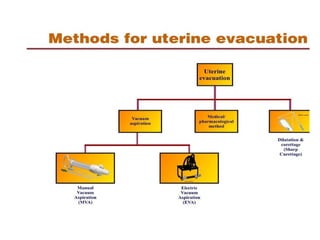
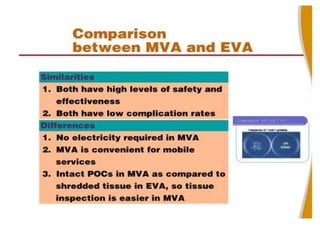
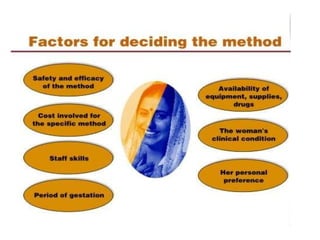
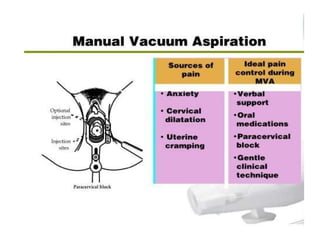
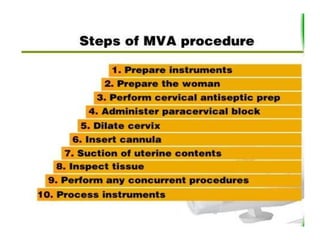
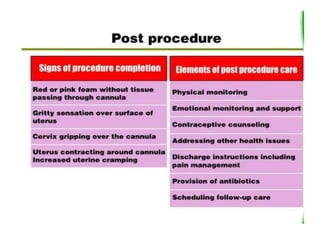
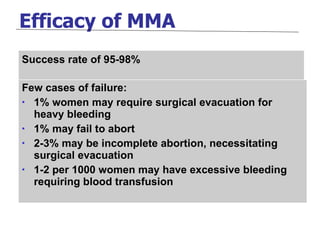
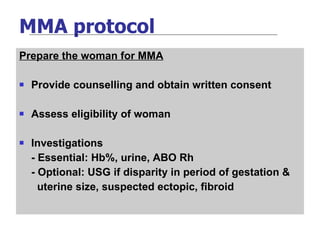
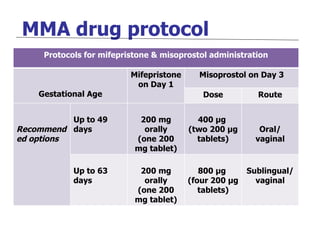
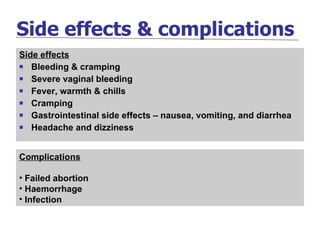
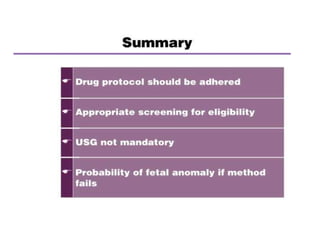
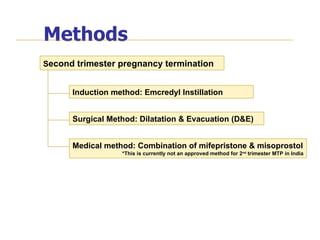
The document discusses family planning and contraceptive use in India. It provides statistics on contraceptive knowledge, use, and methods. The total fertility rate is described. Barriers to meeting contraceptive needs include knowledge gaps, fear of side effects, and limited access to and quality of family planning services. Several contraceptive methods are summarized, including condoms, oral contraceptives, IUDs, and the lactational amenorrhea method.