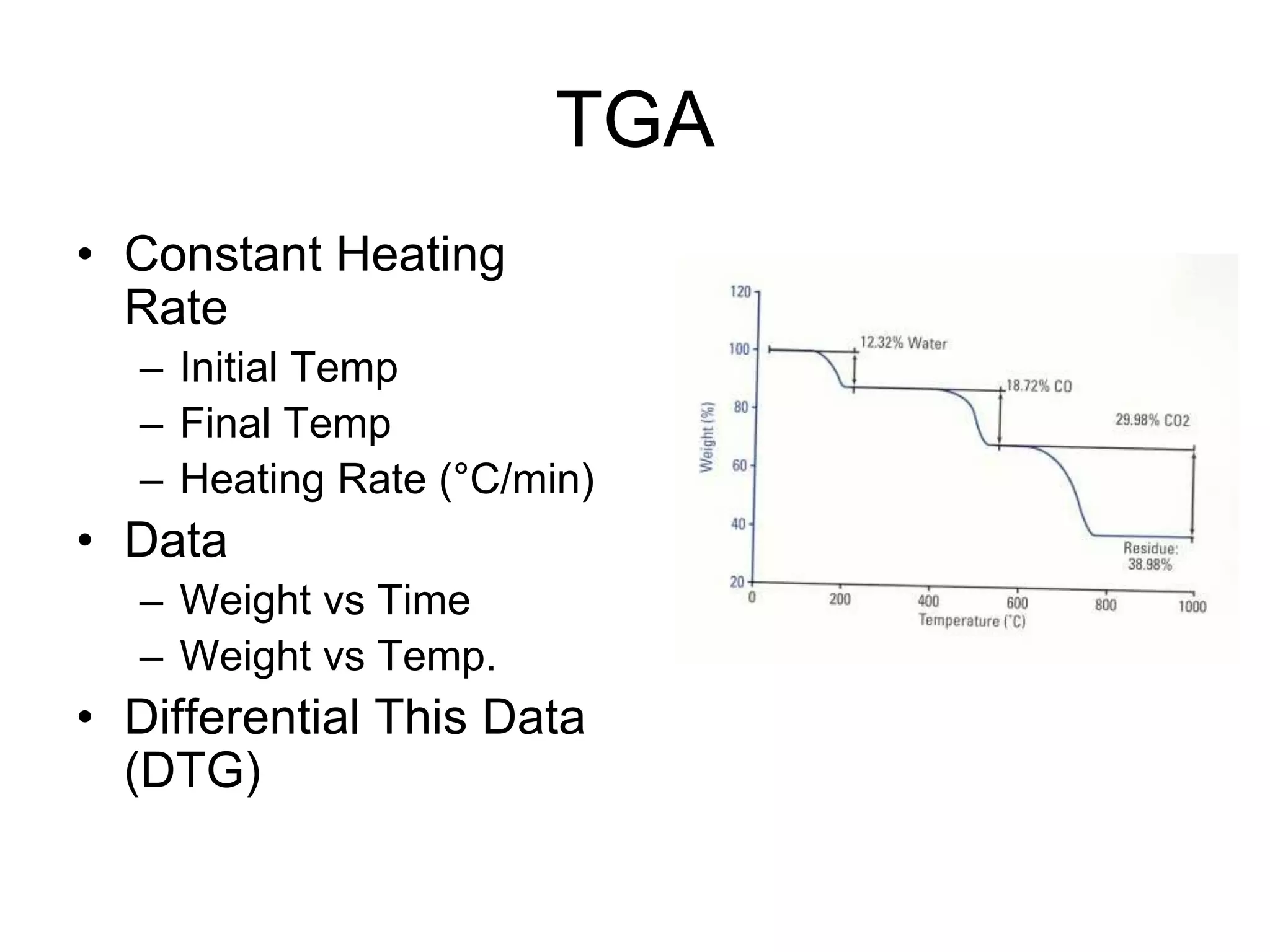
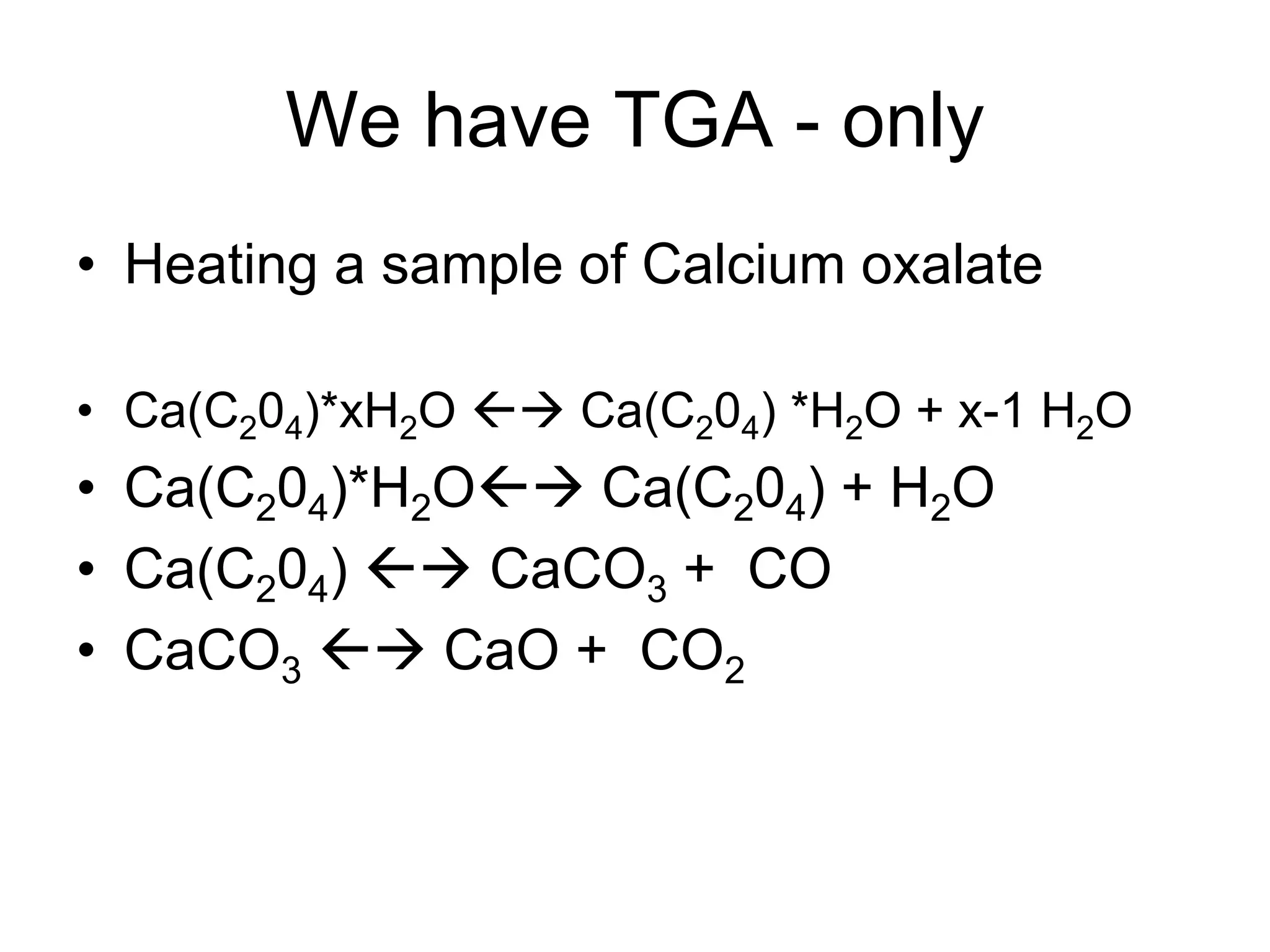
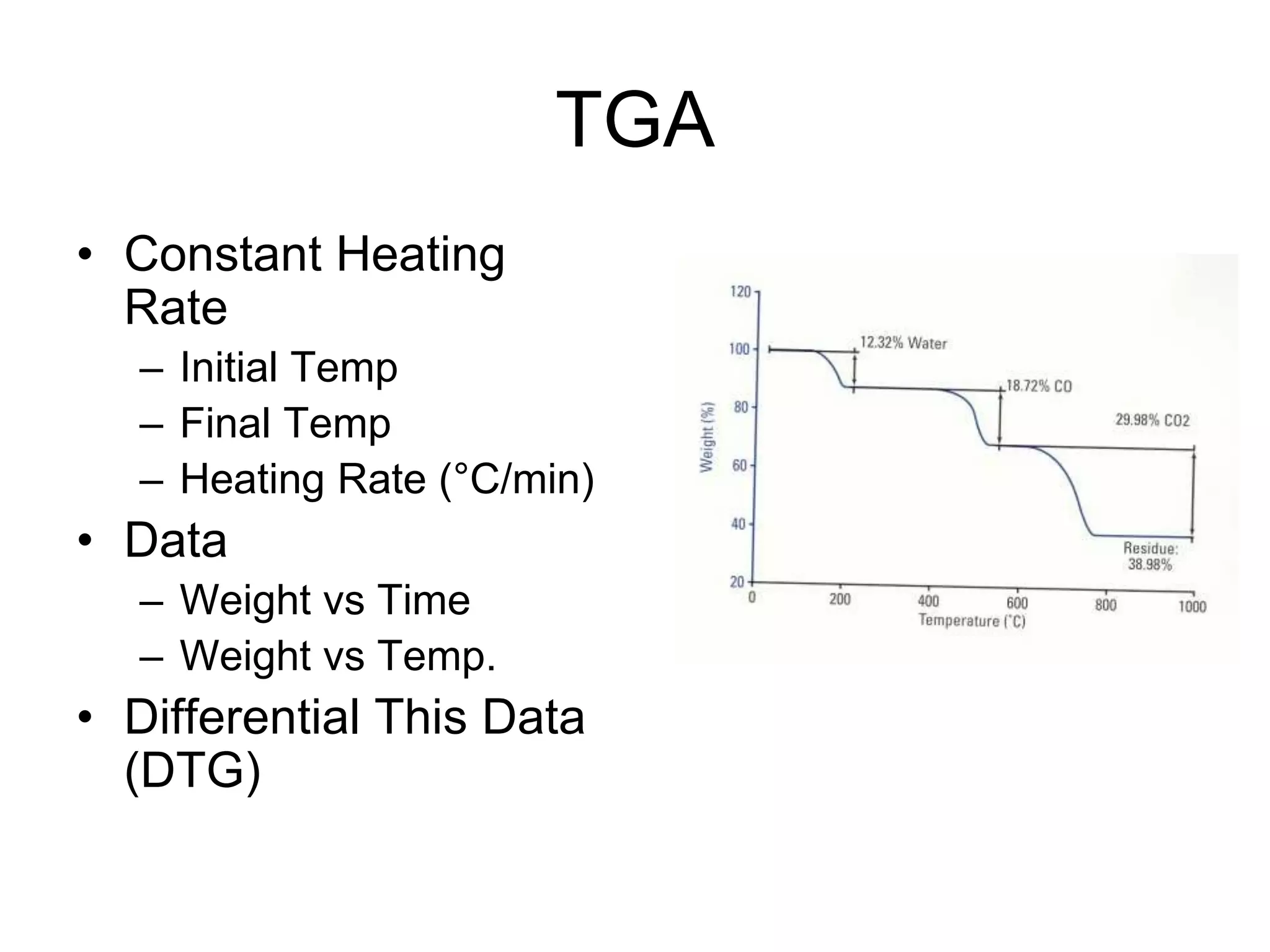
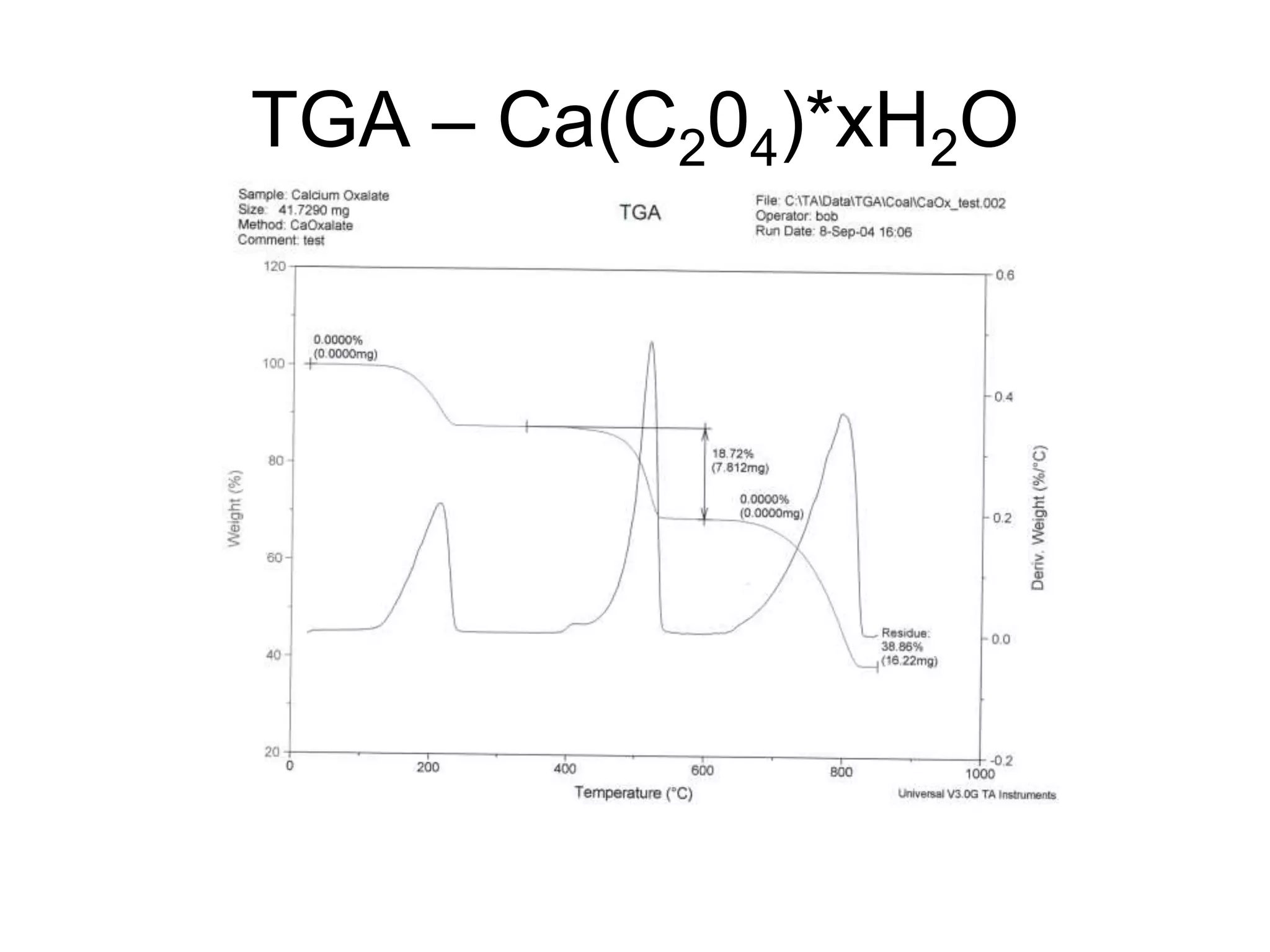
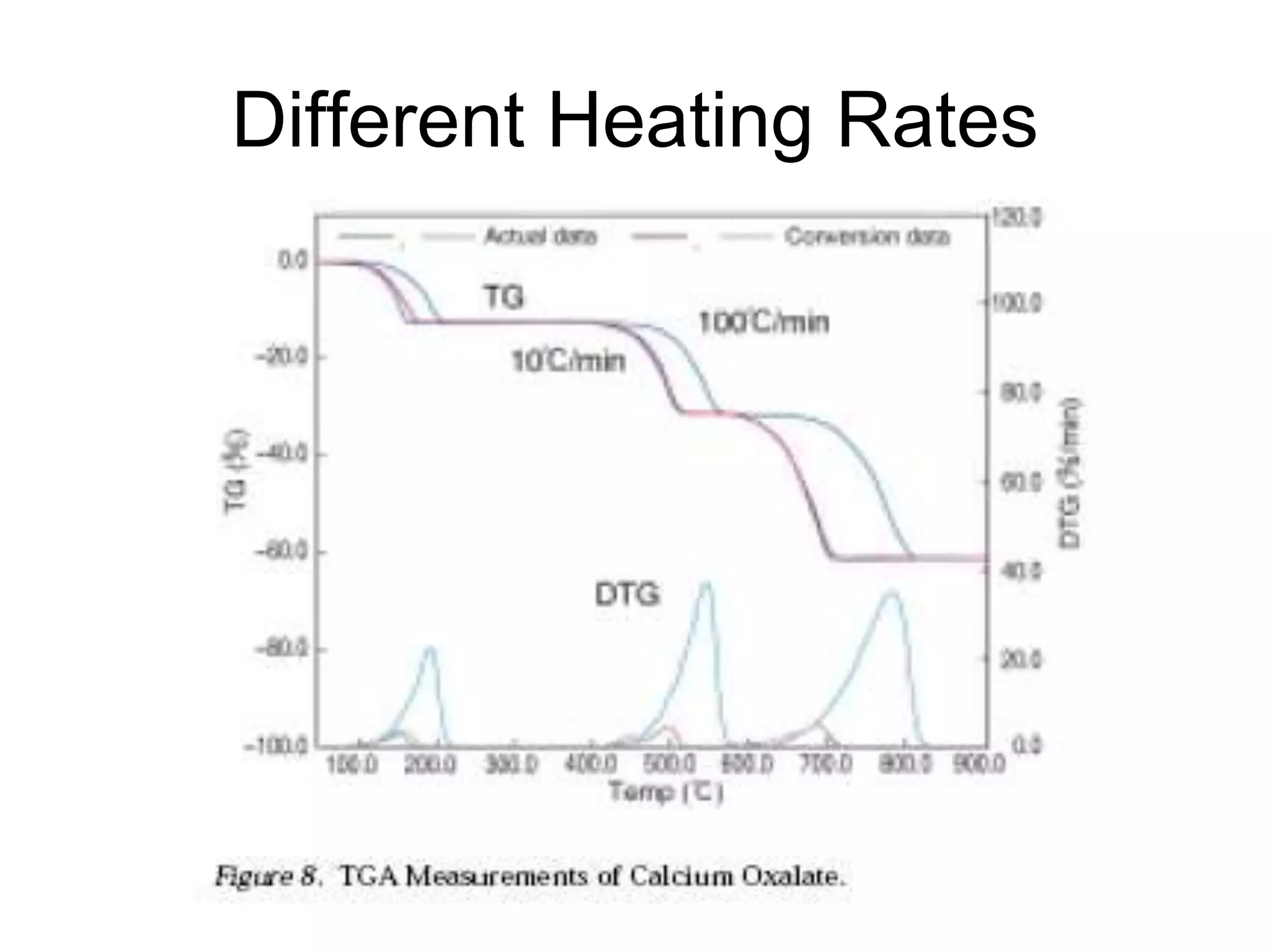
The document discusses various thermal analysis techniques including thermometric titration, thermal mechanical analysis, dynamic mechanical analysis, differential scanning calorimetry, thermal gravimetric analysis, and differential thermal analysis. It provides details on thermal gravimetric analysis and differential thermal analysis, describing the basic principles, data collection, and analysis of results for these methods. An example is given analyzing the thermal decomposition of calcium oxalate dihydrate using thermal gravimetric analysis.