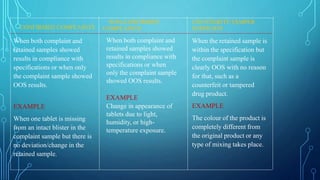
1. The document outlines the standard operating procedure (SOP) for handling product complaints at a pharmaceutical company. It describes the process for receiving, investigating, taking corrective actions for complaints, and generating monthly reports.
2. Key aspects of the SOP include categorizing complaints, timelines for investigation, using a product complaint data sheet to document details, conducting documentation-based and laboratory investigations, and providing feedback and corrective actions to customers.
3. Monthly reports analyze complaint trends to assess quantities and types of complaints received. This allows the company to improve product quality and maintain good customer relationships.