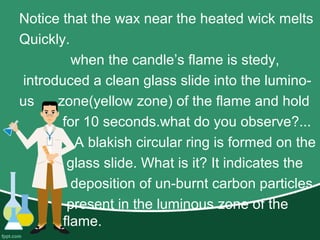
This document provides information about combustion, fuels, and flames. It discusses that combustion is a chemical reaction that generates heat when a material reacts with oxygen. Fuels are materials that burn in combustion and produce heat. Different fuels have different calorific values, or amounts of heat produced during combustion. A flame is produced when a fuel is burned as a gas. A candle flame has distinct color zones - the innermost dark zone where wax vaporizes, a blue zone where vaporized wax burns completely, and an outer luminous yellow zone. The non-luminous zone of the flame is the hottest part.