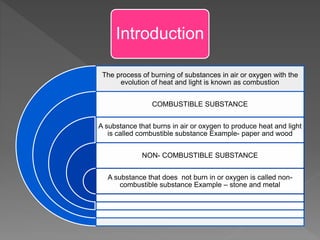
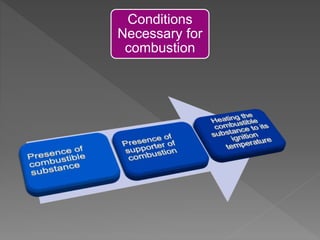
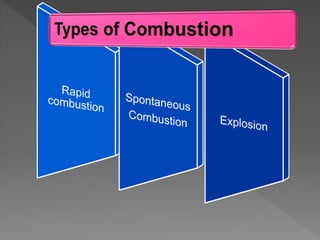
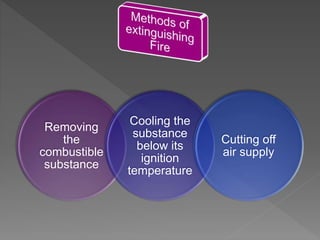
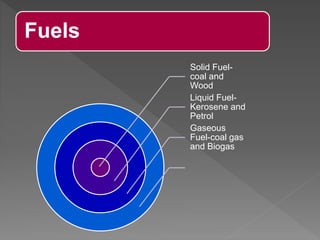
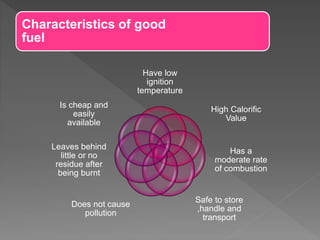
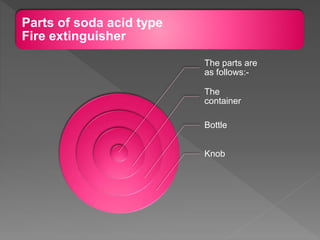
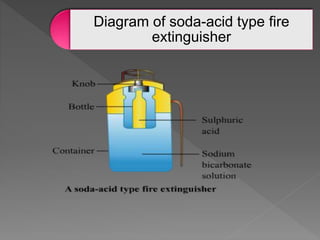
This document provides information about combustion and flames. It defines combustion as the burning of substances in air or oxygen with the evolution of heat and light. Combustible substances burn, while non-combustible substances do not. Three conditions are needed for combustion: oxygen, heat, and a fuel source. Fuels can be solid, liquid, or gas. Good fuels have characteristics like low ignition temperature and high calorific value. The document also describes soda-acid type fire extinguishers, how they work by releasing carbon dioxide gas when the acid bottle breaks, and their parts like the container and knob.