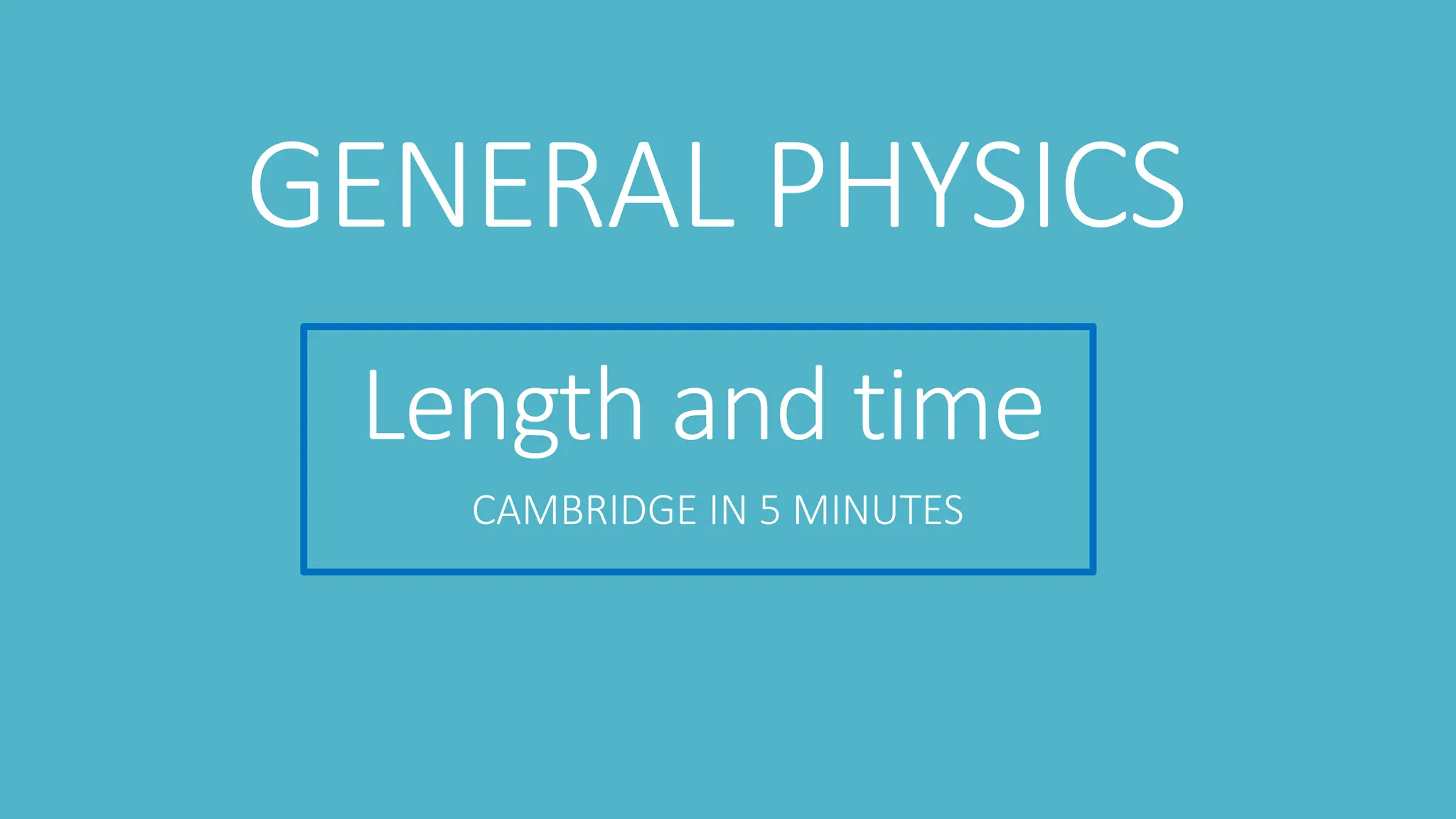
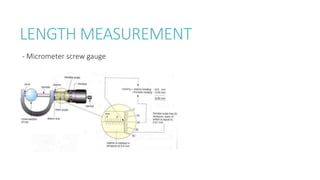
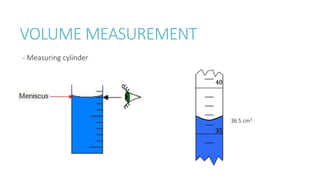
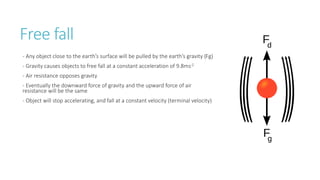
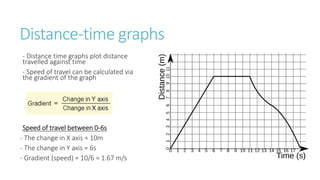
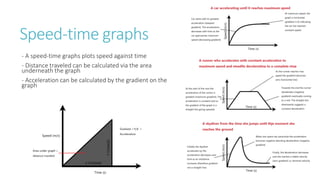
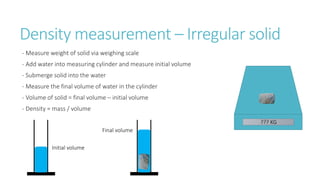
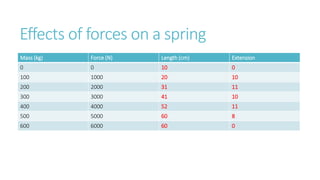
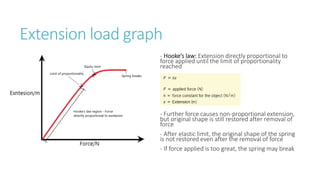
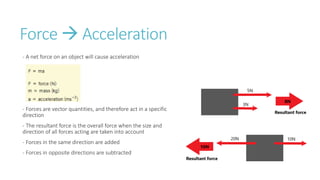
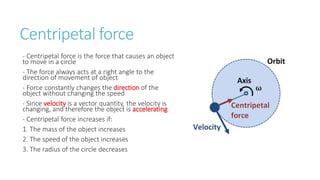
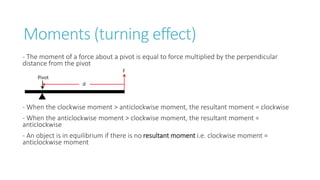
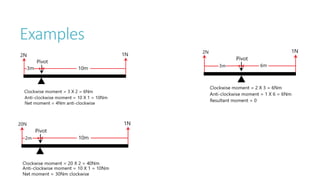
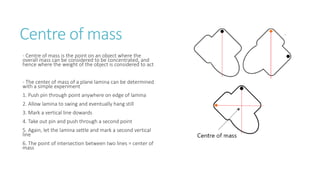
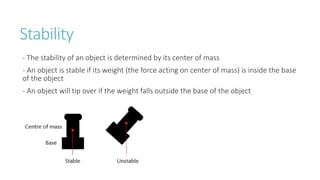
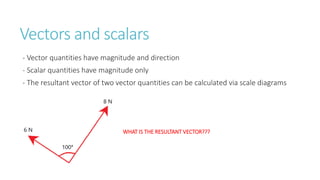
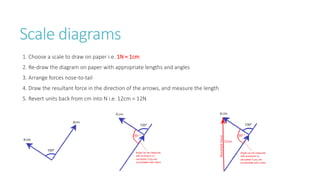
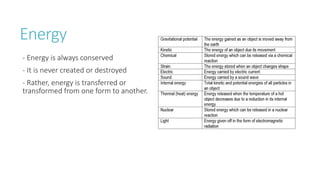
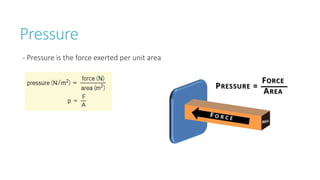
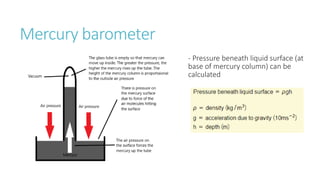
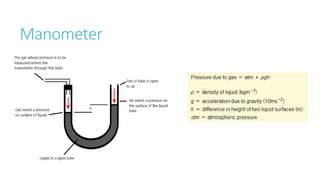
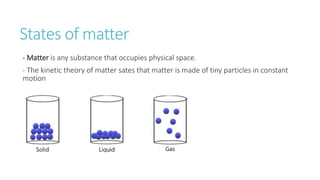
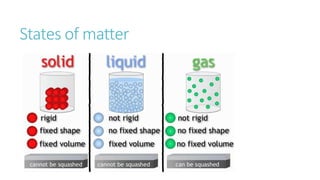
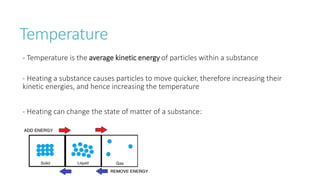
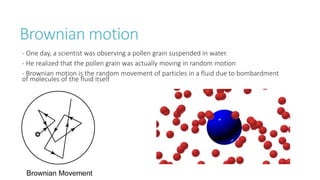
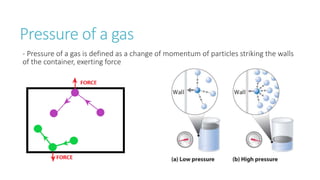
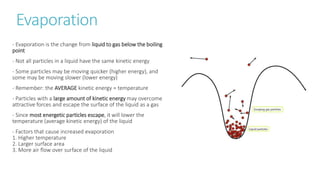
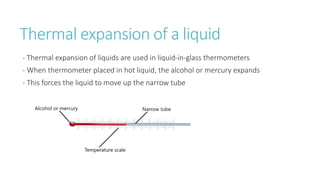
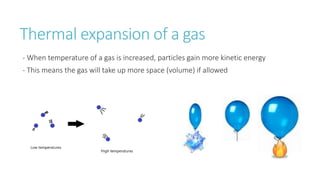
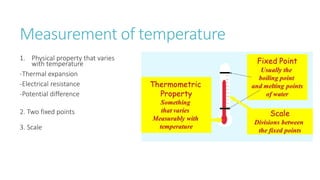
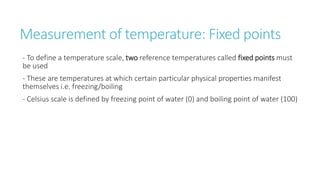
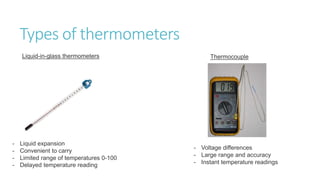
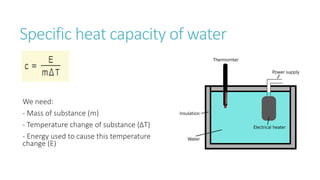
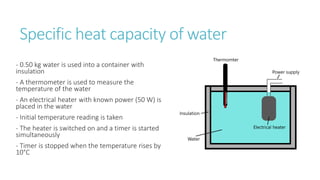
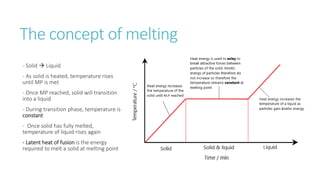
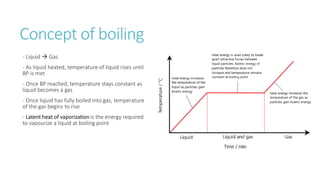
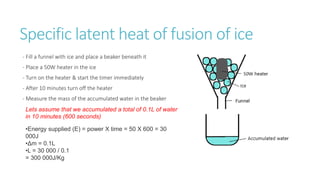
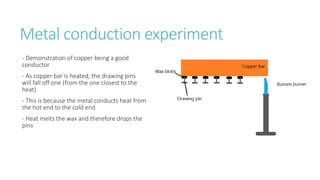
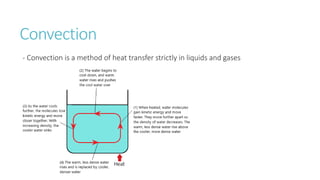
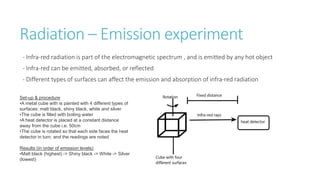
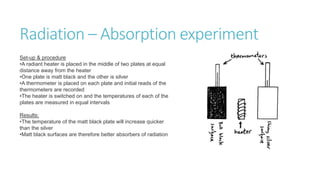
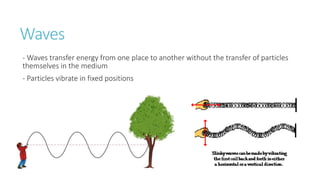
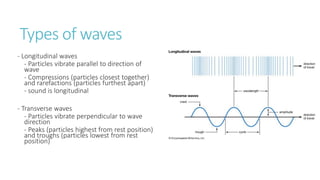
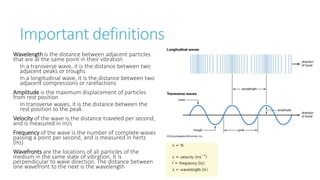
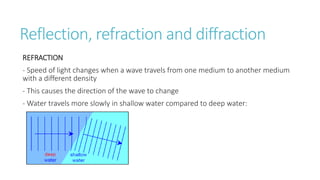
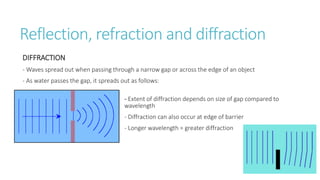
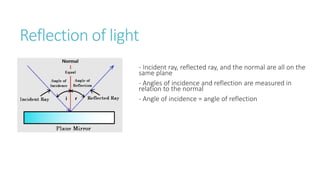
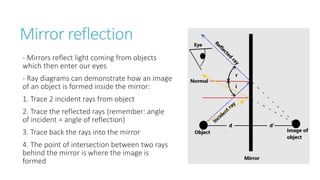
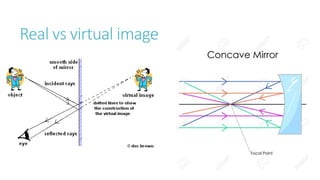
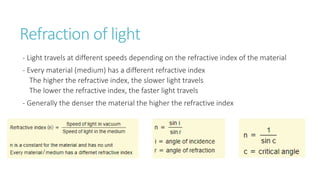
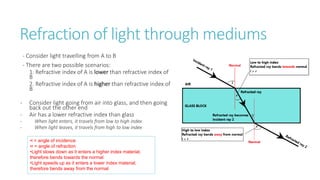
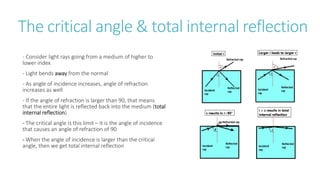
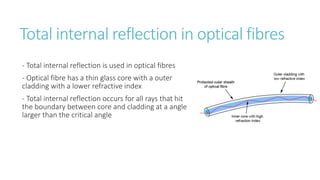
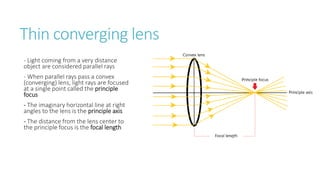
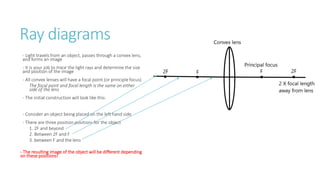
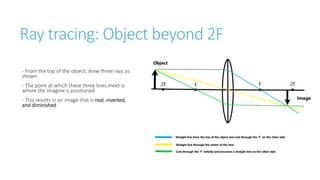
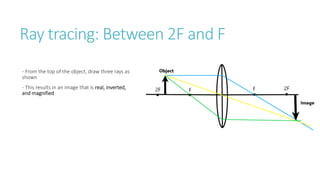
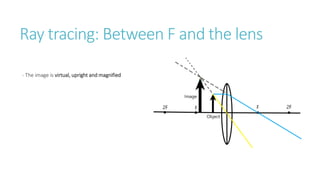
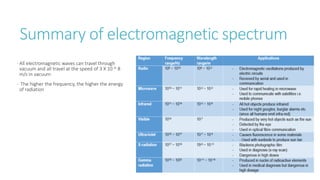
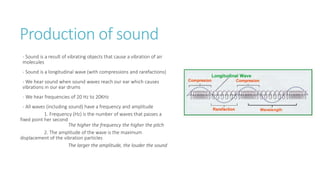
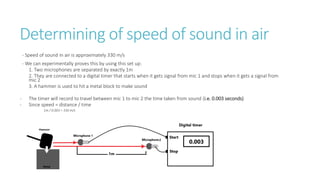
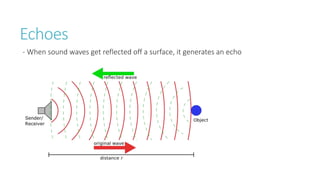
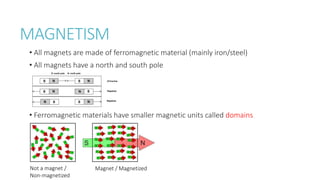
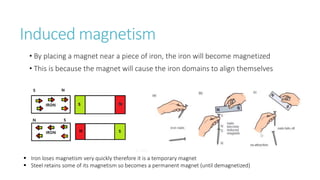
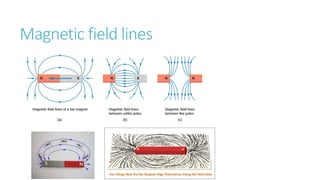
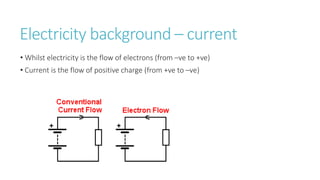
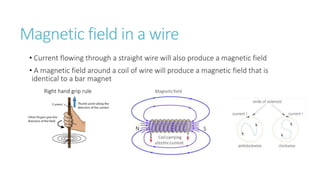
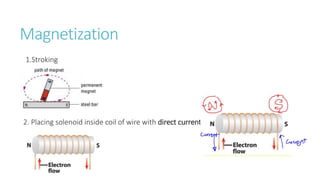
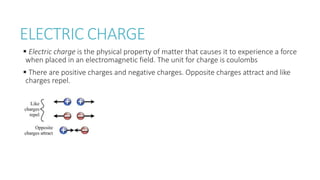
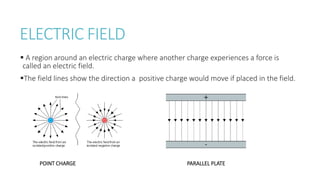
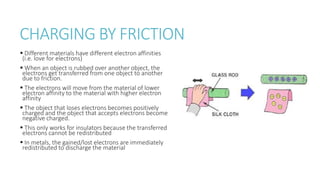
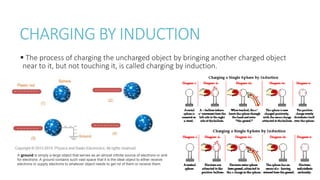
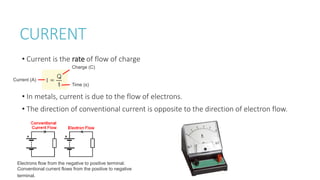
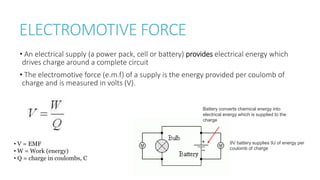
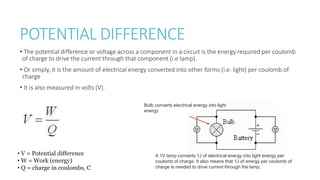
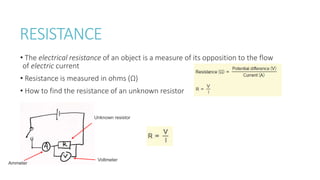
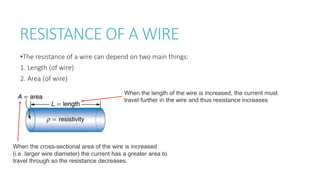
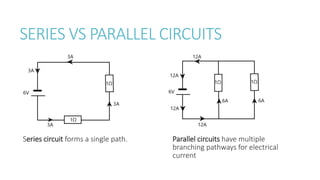
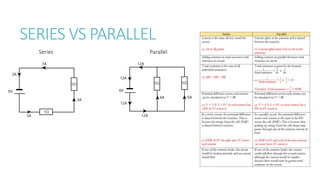
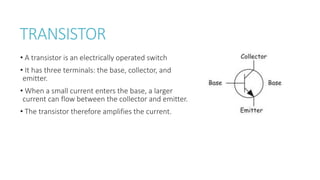
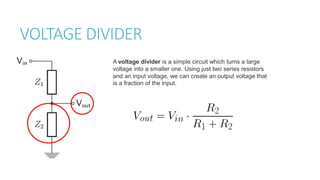
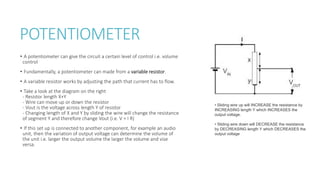
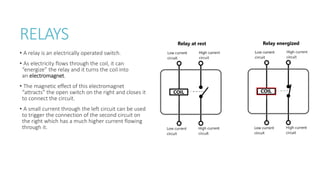
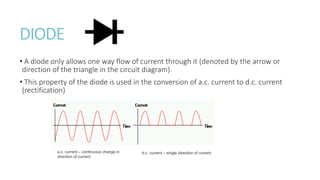
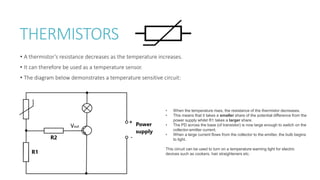
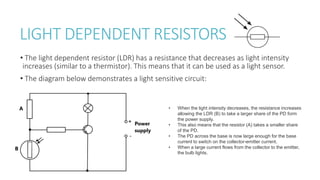
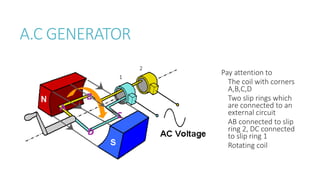
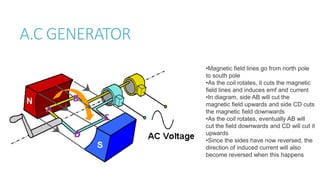
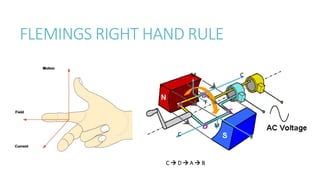
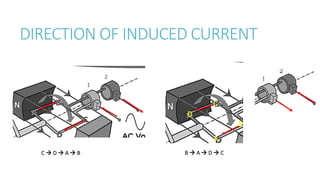
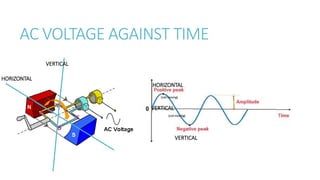
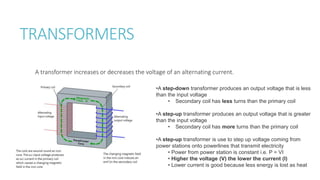
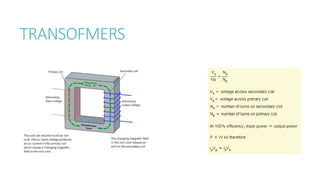
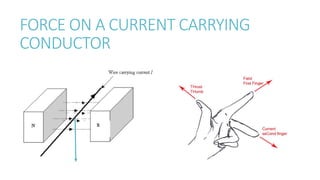
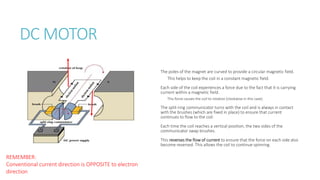
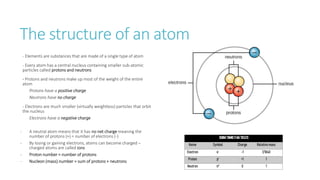
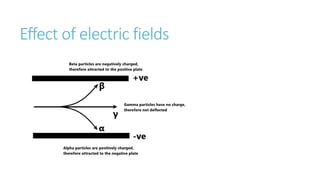
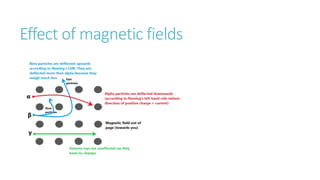
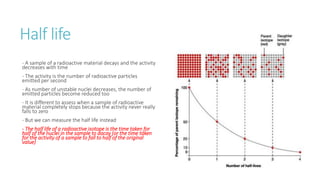
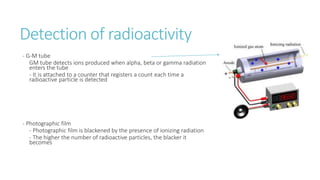
This document provides information on various physics concepts related to length, time, motion, forces, energy, and thermal properties. It defines key terms such as speed, velocity, acceleration, density, mass, weight, pressure, states of matter, temperature, thermal expansion, and specific heat capacity. Measurement techniques are described for length, time, density, forces as well as thermometers. Relationships between various variables are defined through equations and experiments. Overall, the document serves as a review of basic physics topics.