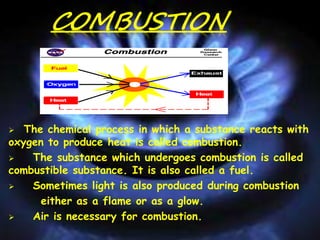
The document defines combustion as the chemical process where a substance reacts with oxygen to produce heat. It requires three conditions: a fuel, oxygen from air, and heat. Combustible substances that burn are called fuels. Combustion can be spontaneous or explosive. Fire is controlled by methods like using water or carbon dioxide to cut off the oxygen supply or reduce heat. A flame zone forms when fuels vaporize during burning and has inner, middle, and outer zones of different temperatures and colors.