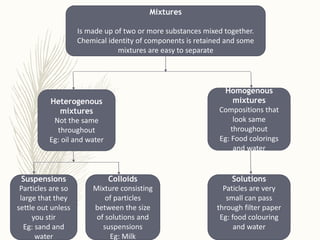
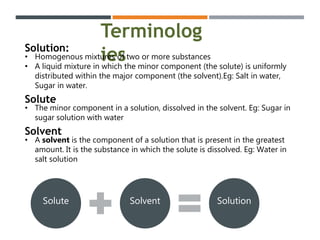
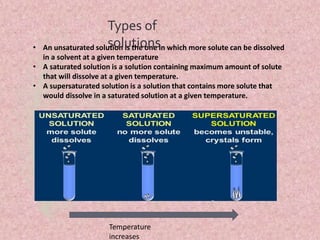
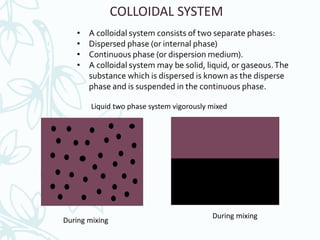
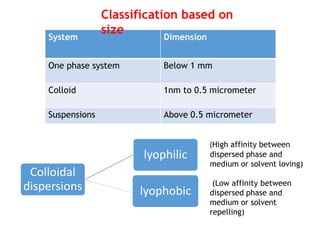
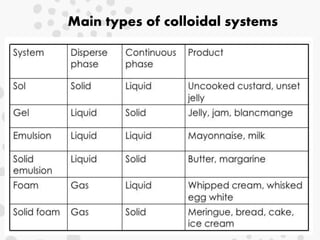
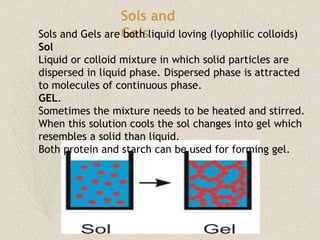
This document discusses colloidal systems found in food. It begins by defining mixtures, solutions, suspensions, and colloids. A colloid is a mixture with particle sizes between solutions and suspensions. Common colloidal systems in food include sols, gels, and emulsions. Sols have solid particles dispersed in a liquid, while gels are solidified sols. Emulsions disperse one liquid into another immiscible liquid. Food colloids are important for texture, structure, stability, and maintaining properties of food systems.