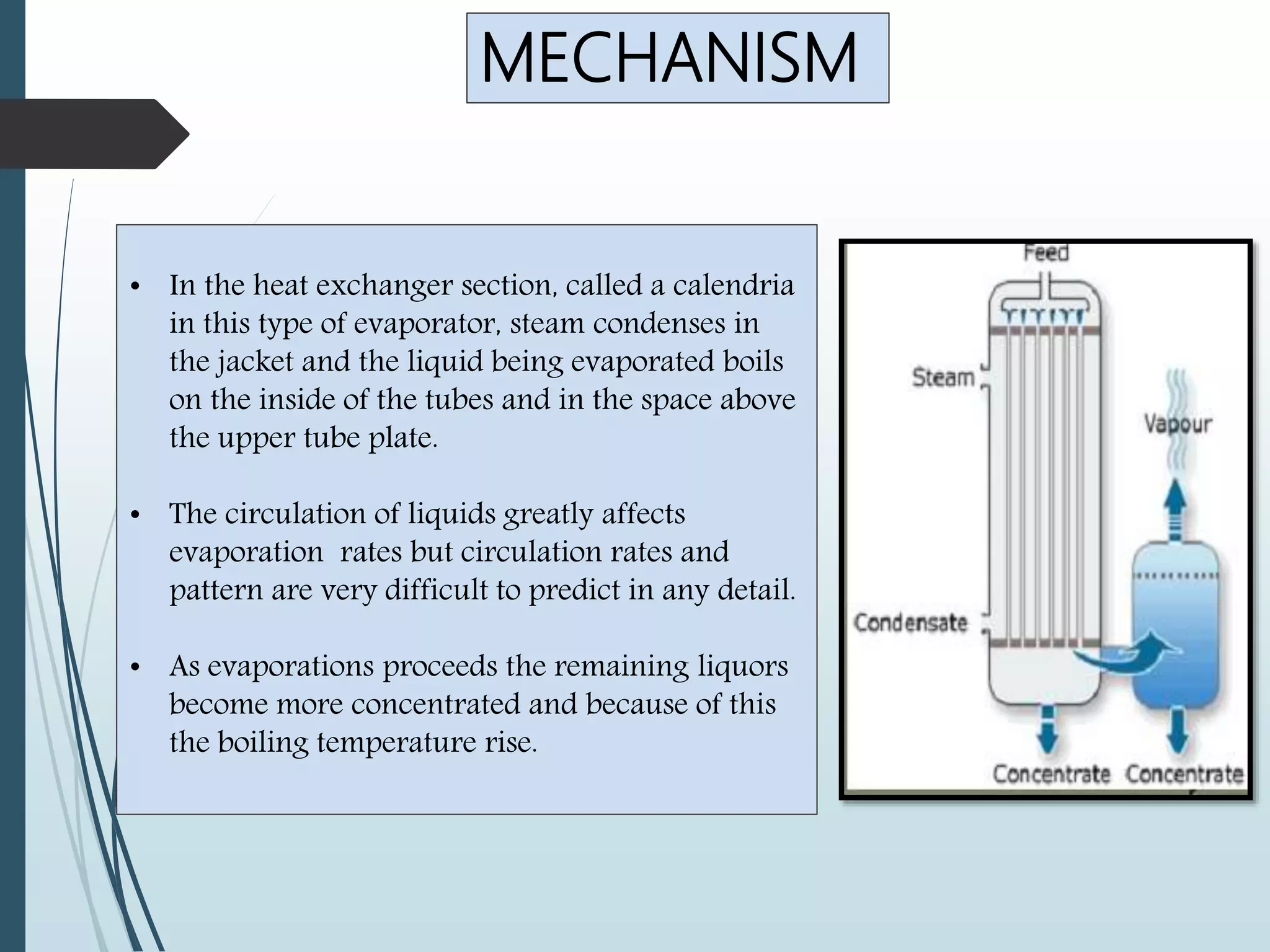
Evaporation is a process used in the food industry to remove water from raw materials or foodstuffs to produce a more concentrated final product. It involves heating a liquid to vaporize its water content. Some key points:
- Evaporation is used extensively in concentrating fruit and vegetable juices, milk, coffee extracts and in refining sugar and salt.
- It reduces the weight, volume, storage and transportation costs of products while improving storage stability.
- Multiple effect evaporators are more energy efficient as they use steam from one chamber to heat the next, allowing more water to be evaporated with the same amount of steam.
- Food quality must be maintained during evaporation to minimize losses of nutrients