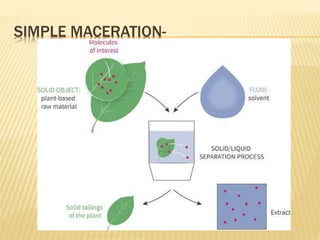
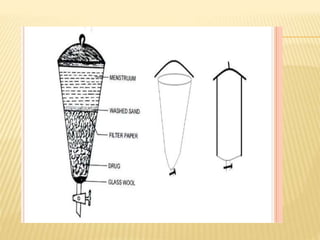
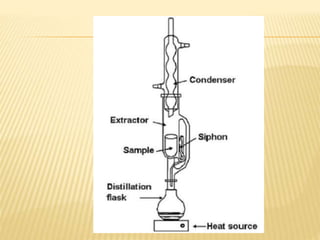
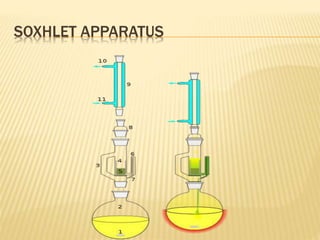
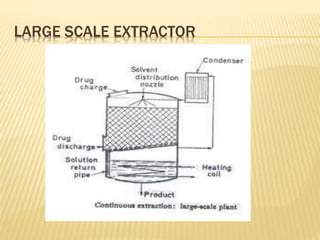
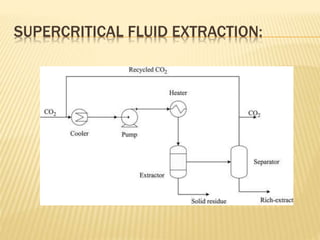
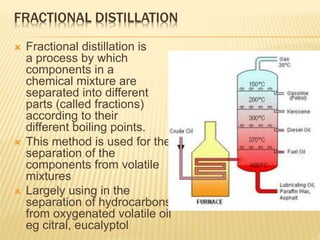
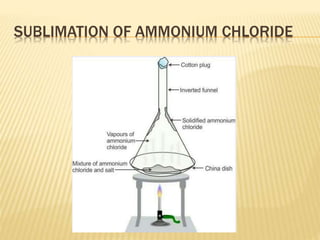
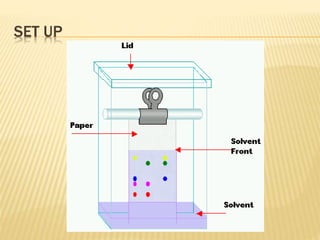
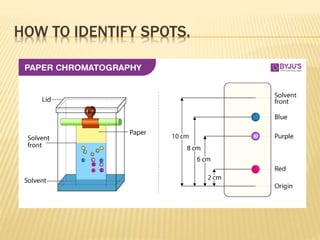
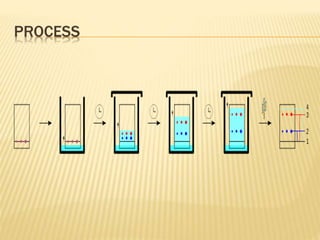
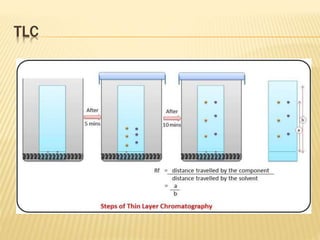
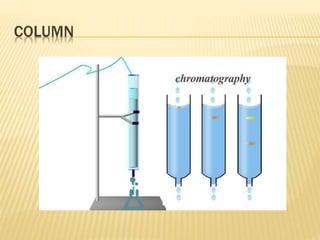
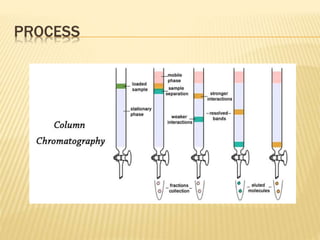
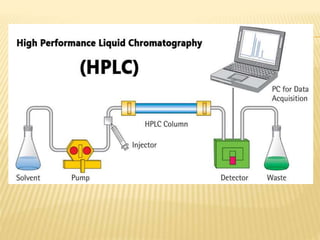
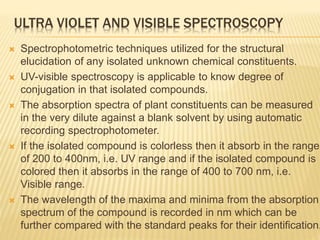
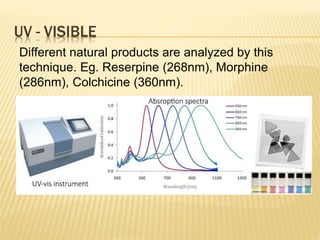
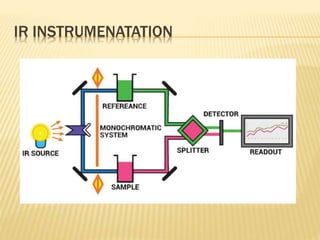
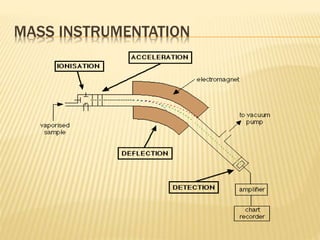
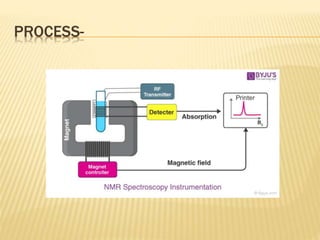
The document discusses various concepts and techniques used in phytochemistry including modern extraction methods like maceration, percolation, Soxhlet extraction and supercritical fluid extraction. It also covers isolation and purification techniques like fractional crystallization, distillation and sublimation. Methods of separation like paper chromatography, thin layer chromatography, gas chromatography and various spectroscopy techniques for identification are summarized.