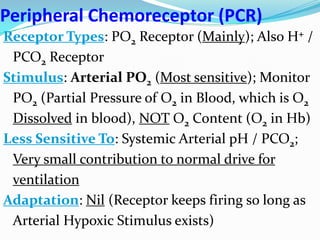
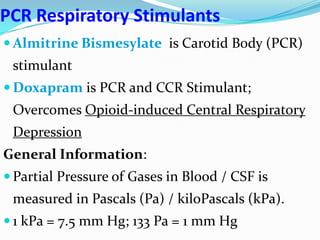
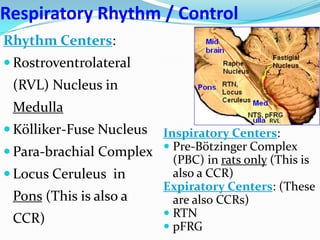
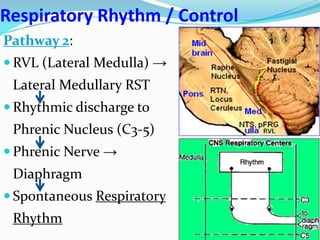
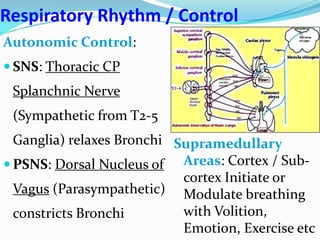
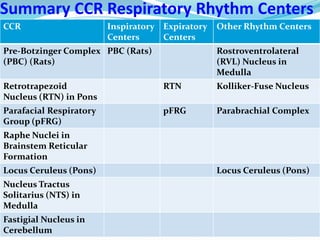
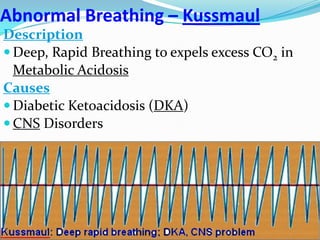
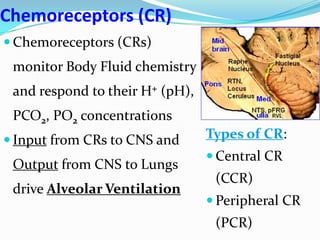
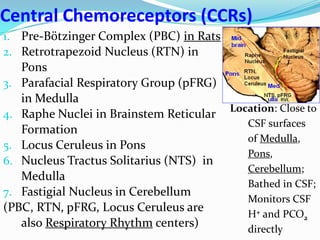
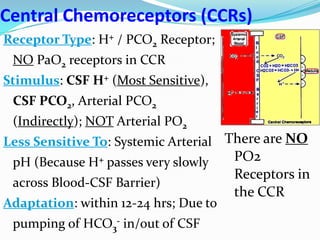
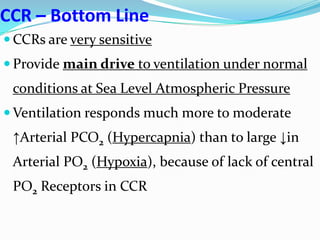
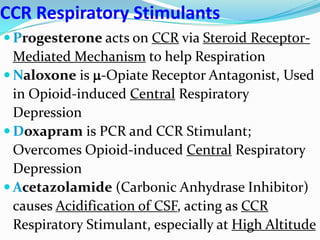
The document provides an in-depth overview of central and peripheral chemoreceptors (CCR and PCR) involved in regulating respiration, detailing their locations, functions, and key stimulants. It discusses the role of these receptors in monitoring blood gas levels, how they influence ventilation in response to changes in carbon dioxide and oxygen levels, and highlights the mechanisms leading to respiratory control. Additionally, the document touches on various clinical aspects related to respiratory control abnormalities, types of abnormal breathing patterns, and the effects of certain stimulants on respiration.

![Peripheral Chemoreceptors (PCR)
Locations: Aortic and Carotid Bodies;
Bathed in Arterial Blood; Monitor
Arterial Blood PO2 directly
1. Aortic Bodies: Near Aortic Arch;
CN10 Afferent
2. Carotid Bodies (Most important):
Near Carotid Sinus at Carotid
Bifurcation; CN9 Afferent
[Very small structure; Receives maximum
Blood per Gm of tissue; Meets metabolic
requirements by utilizing O2 dissolved in
Blood; Type 1 Glomus Cells are main
sensors of Hypoxia]](https://image.slidesharecdn.com/xcnscontrolofrespirationss-140316192823-phpapp01/85/Neural-Control-of-Respiration-Abnormal-Breathing-Patterns-Sanjoy-Sanyal-7-320.jpg)