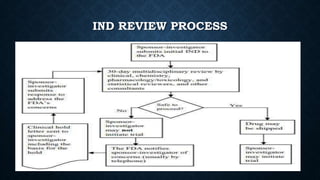
Clinical trials involve several phases of research to evaluate new drugs and therapies in humans. Phase 1 trials involve small groups of people to determine safety. Phase 2 trials administer the therapy to groups of patients to identify effective doses and further evaluate safety. Phase 3 trials involve large groups of patients and are designed to confirm effectiveness. After approval, Phase 4 trials monitor long-term safety and effectiveness in broader patient populations. Each phase helps researchers and regulators determine whether new therapies should advance in development or be approved for medical use.