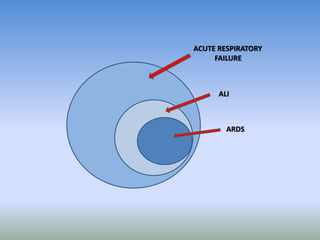
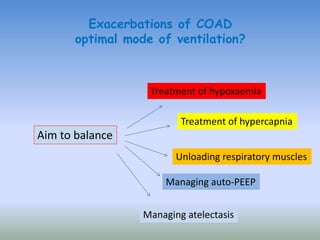
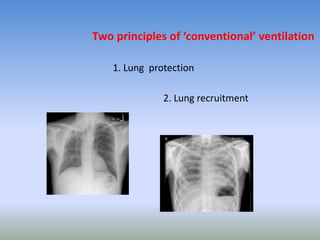
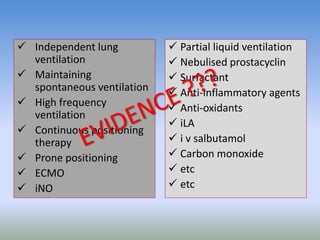
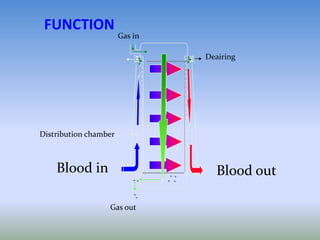
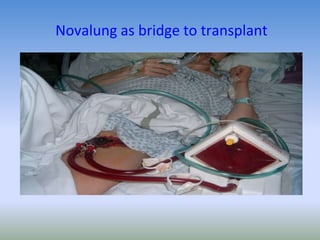
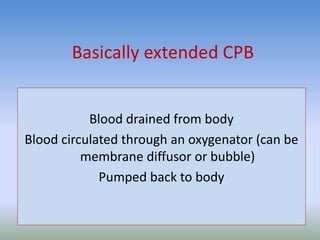
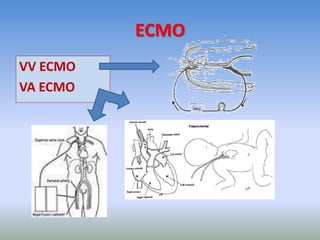
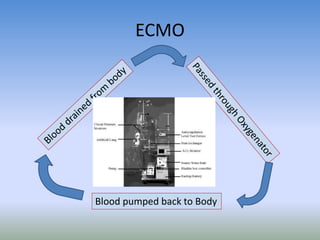
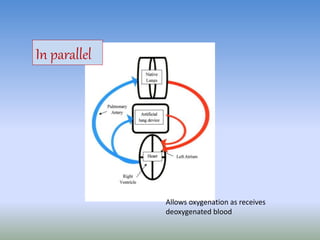
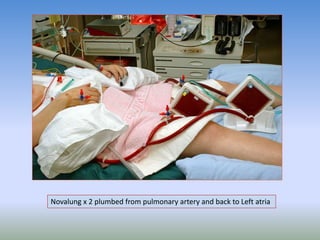
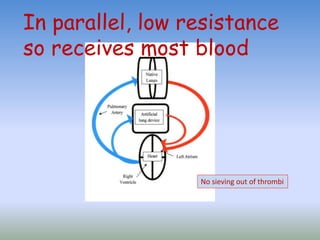
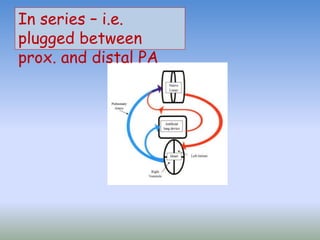
This document discusses various treatments for chronic respiratory failure, including different modes of ventilation and adjunctive therapies. It covers topics like treating hypoxemia, hypercapnia, and unloading respiratory muscles. Newer technologies discussed include high frequency oscillatory ventilation, various forms of extracorporeal membrane oxygenation (ECMO), and potential future treatments like artificial lungs or stem cell therapy. The key principles of treatment are protecting the lungs from further injury while also recruiting functional but non-functioning lung tissue through various positioning techniques or devices.