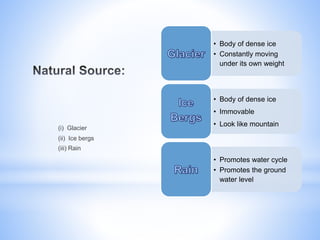
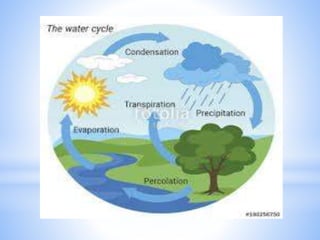
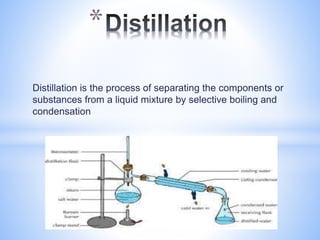
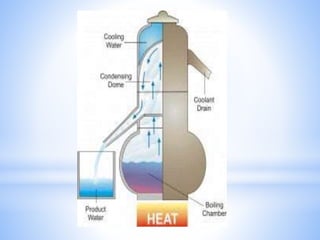
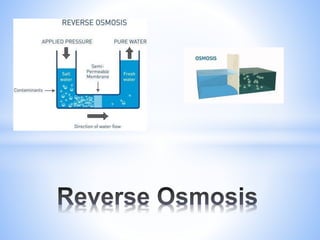
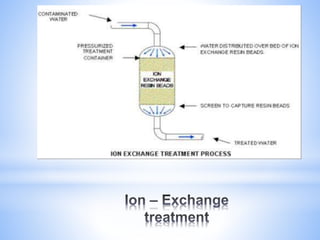
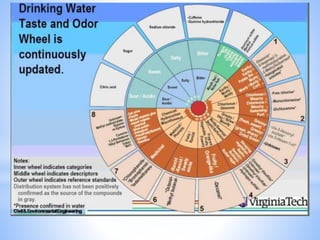
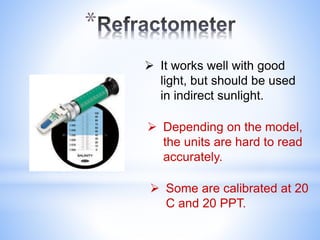
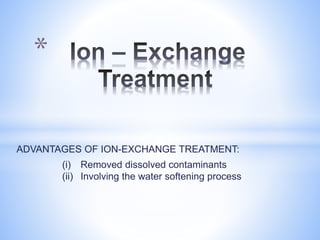This document provides information about analyzing water quality parameters. It discusses various methods for testing parameters like pH, dissolved oxygen, salinity, and others. Colorimetric analysis using a colorimeter or photometer that measures light wavelengths absorbed by chemical reactions is described as one useful method. The LaMotte SMART 3 colorimeter is highlighted as a computerized tool that can conduct multiple water quality tests by analyzing the intensity of light for different chemical reactions.