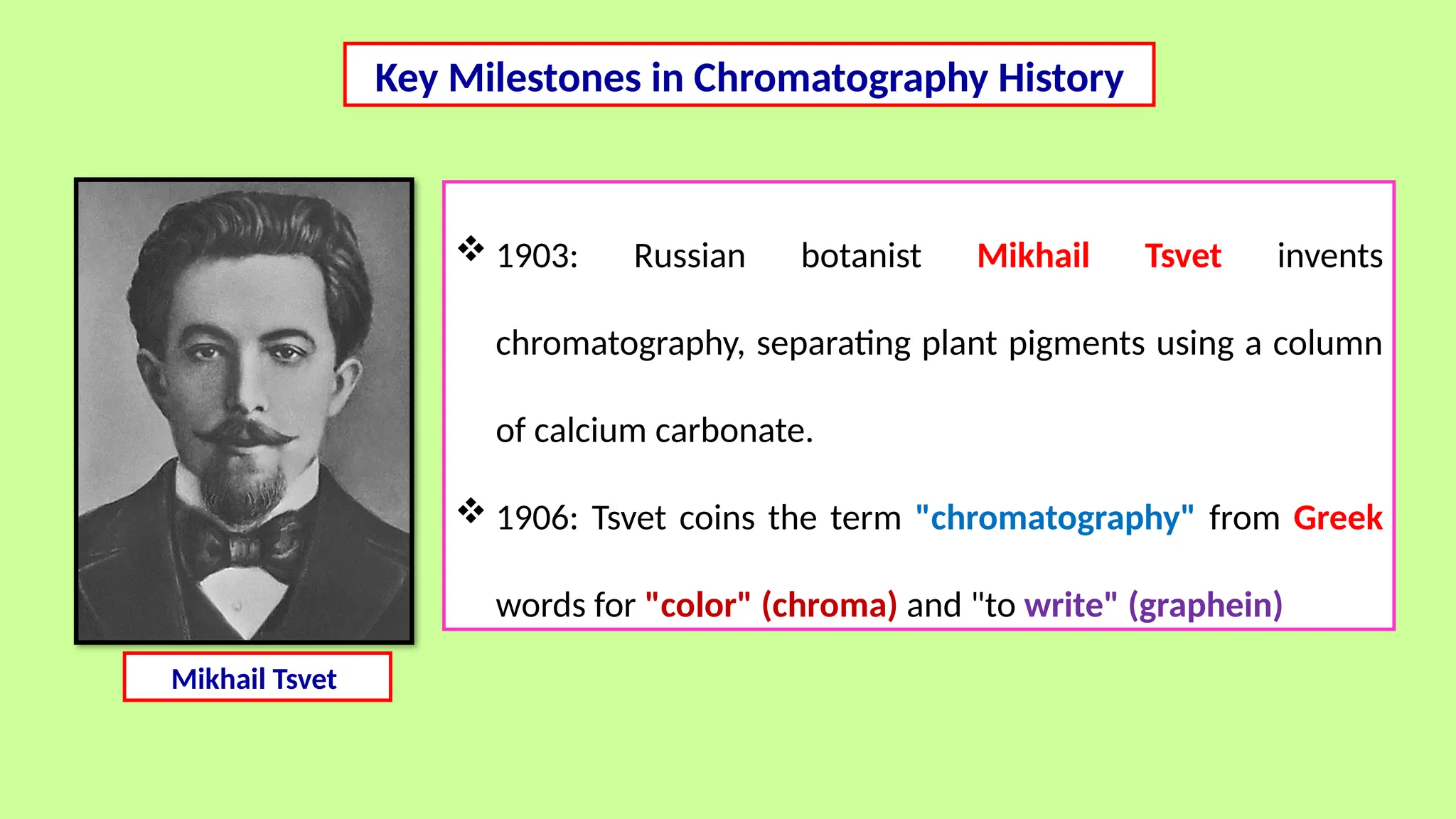
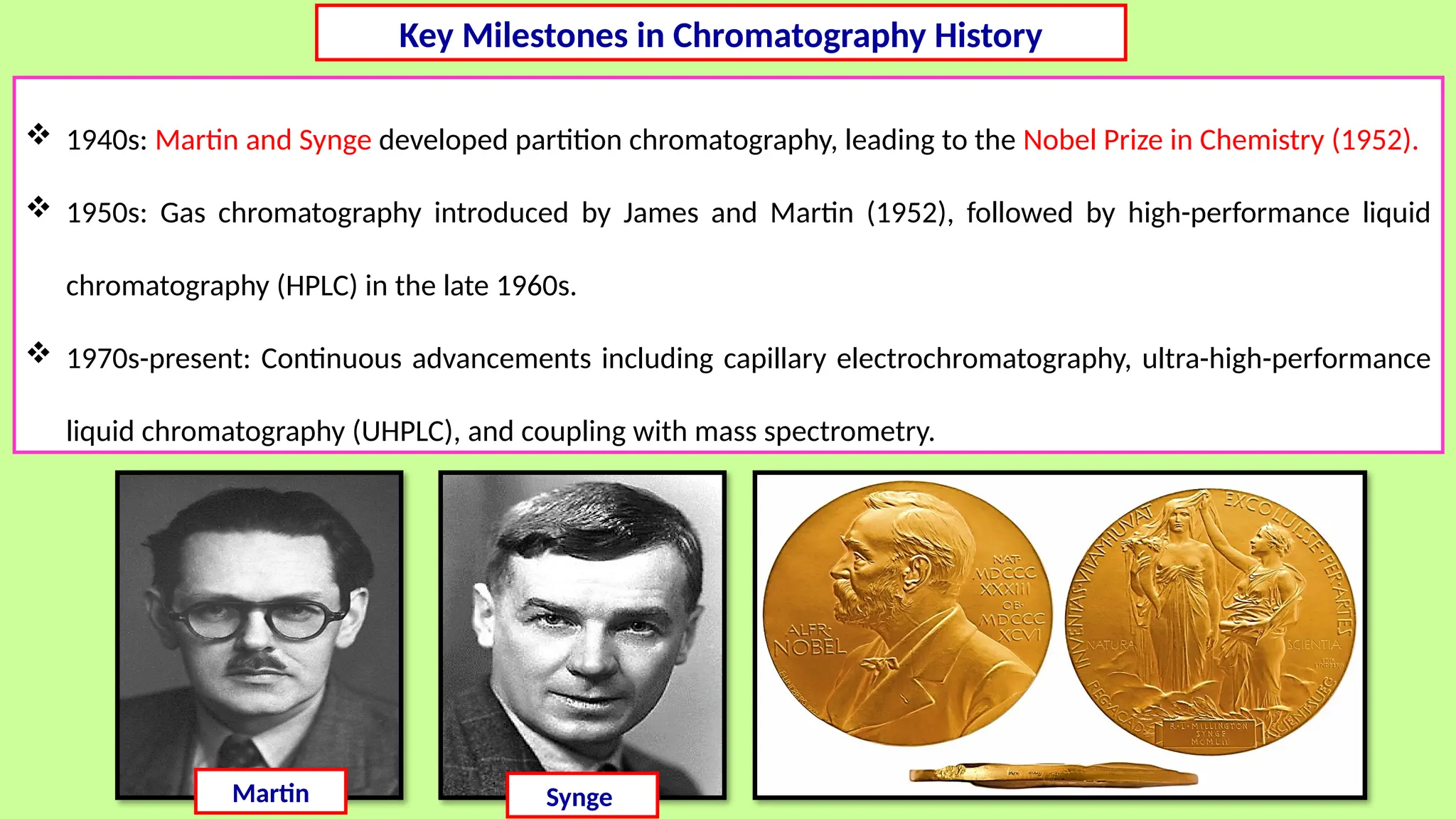
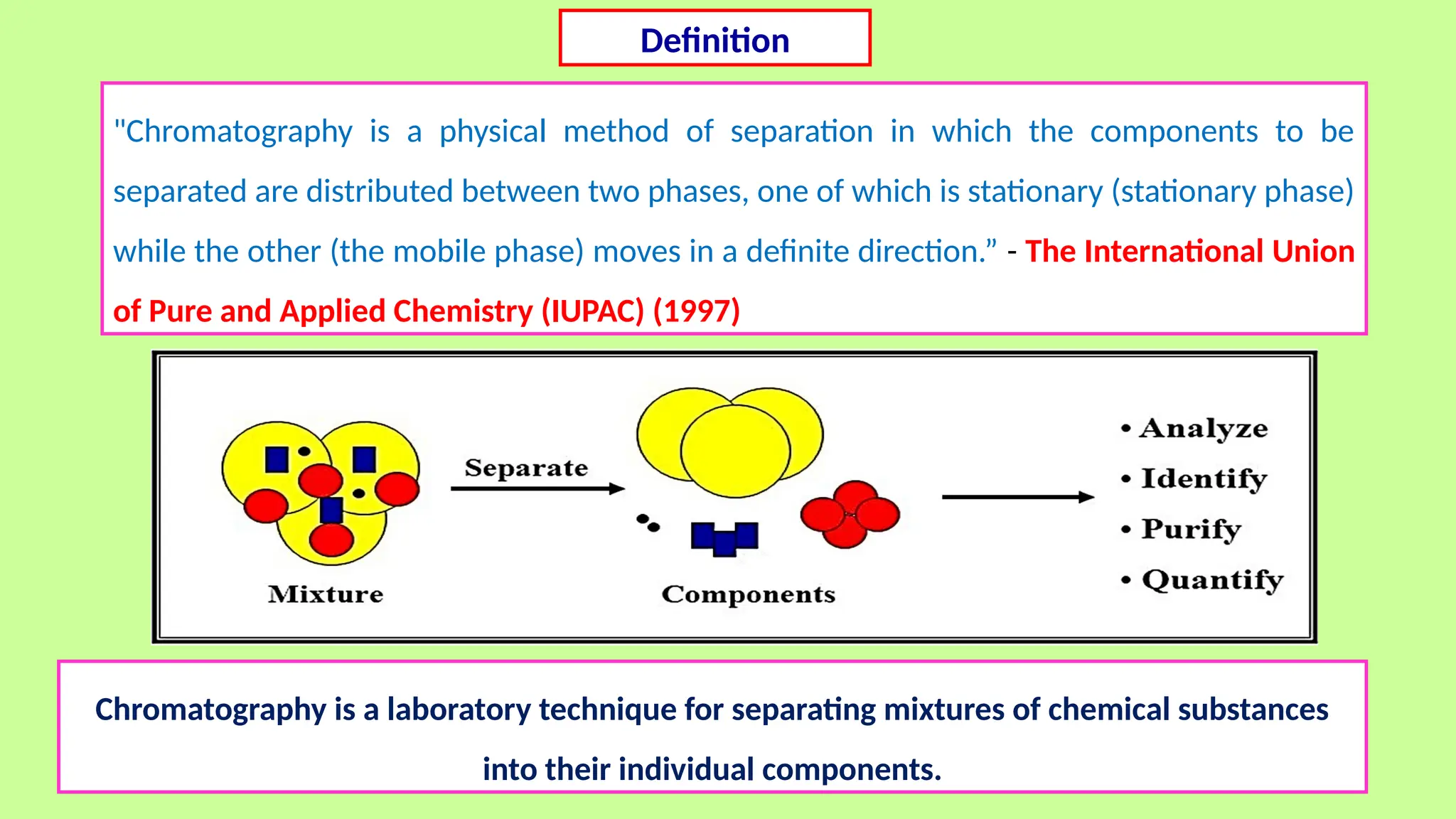
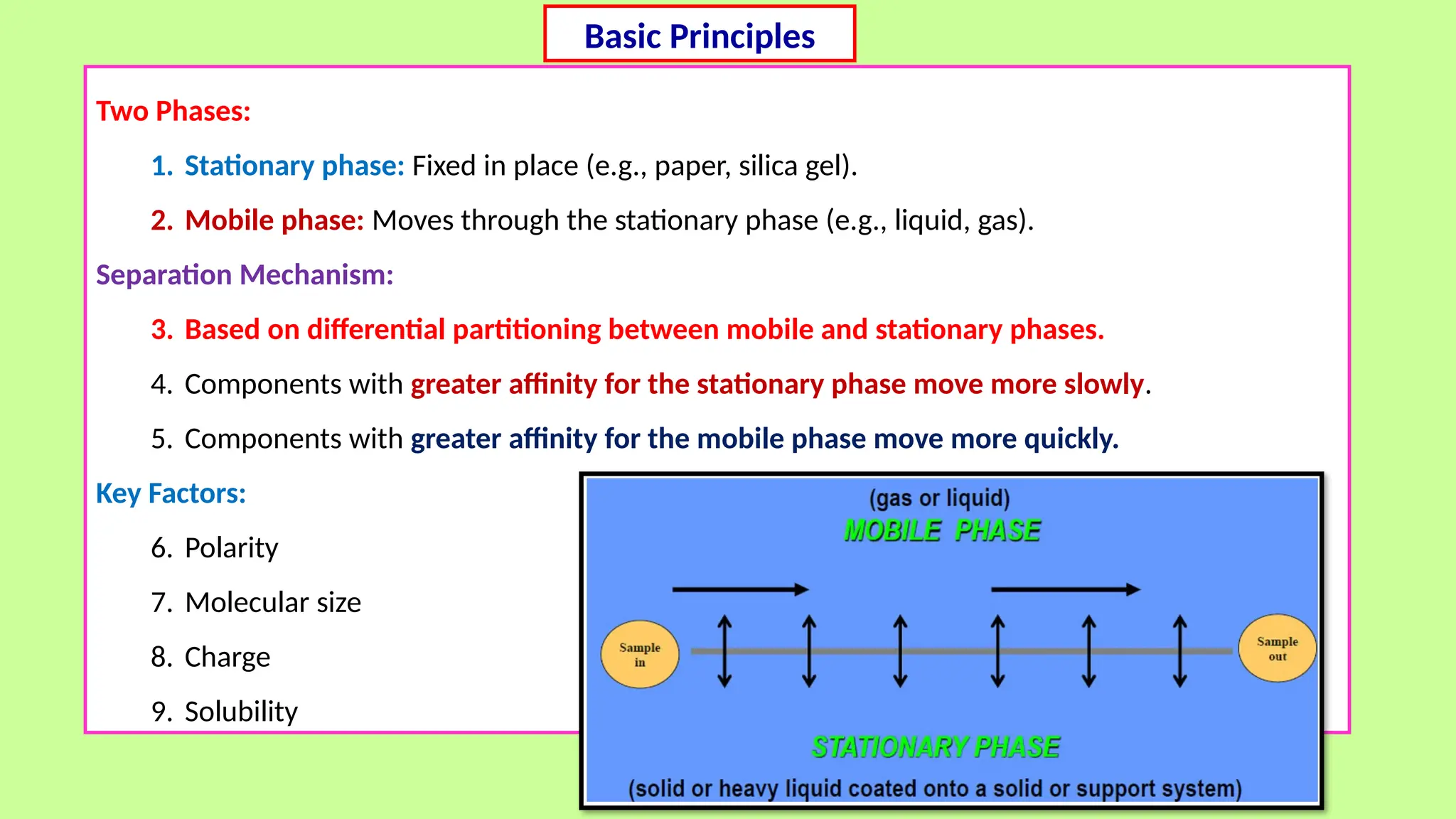
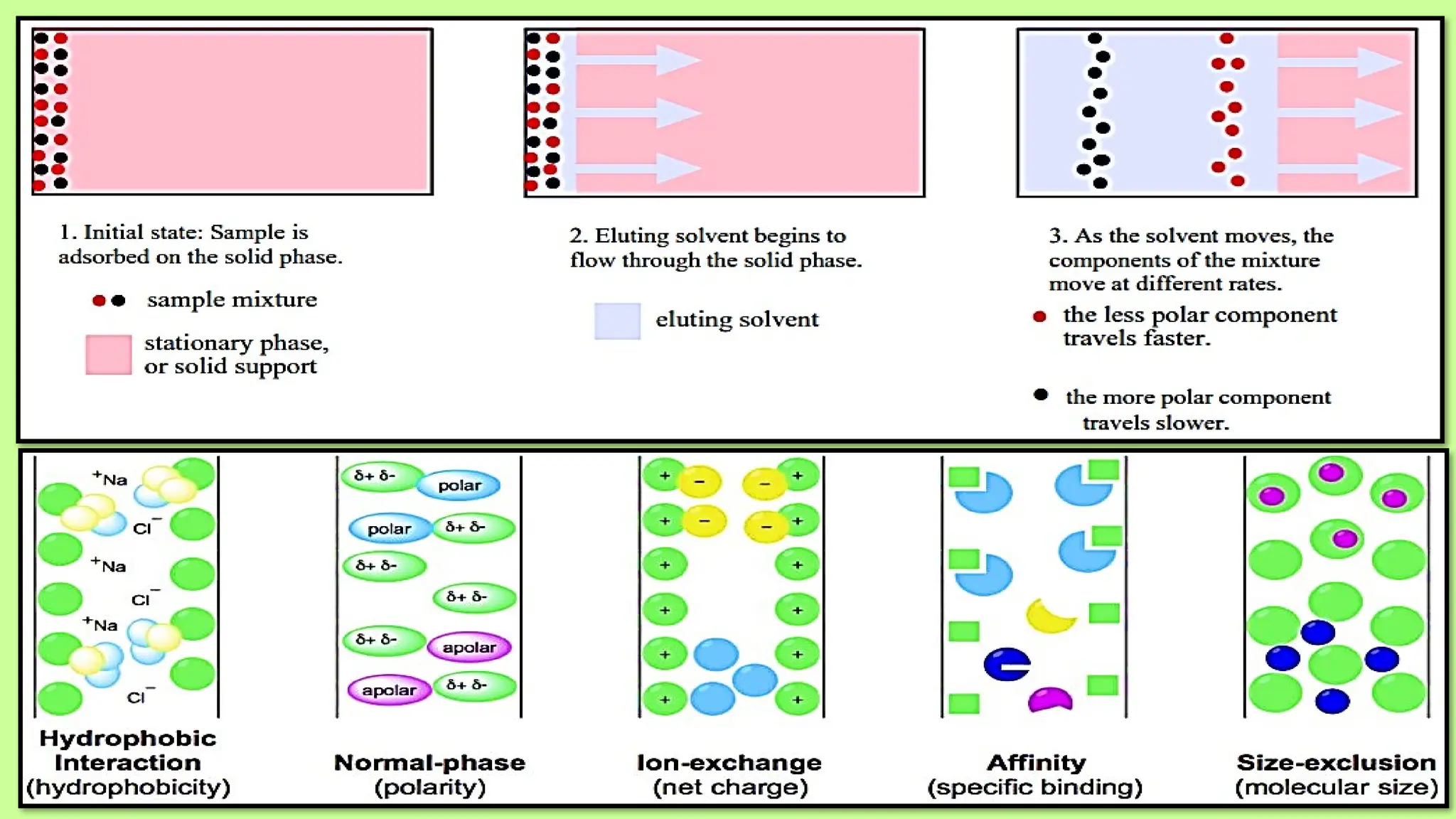
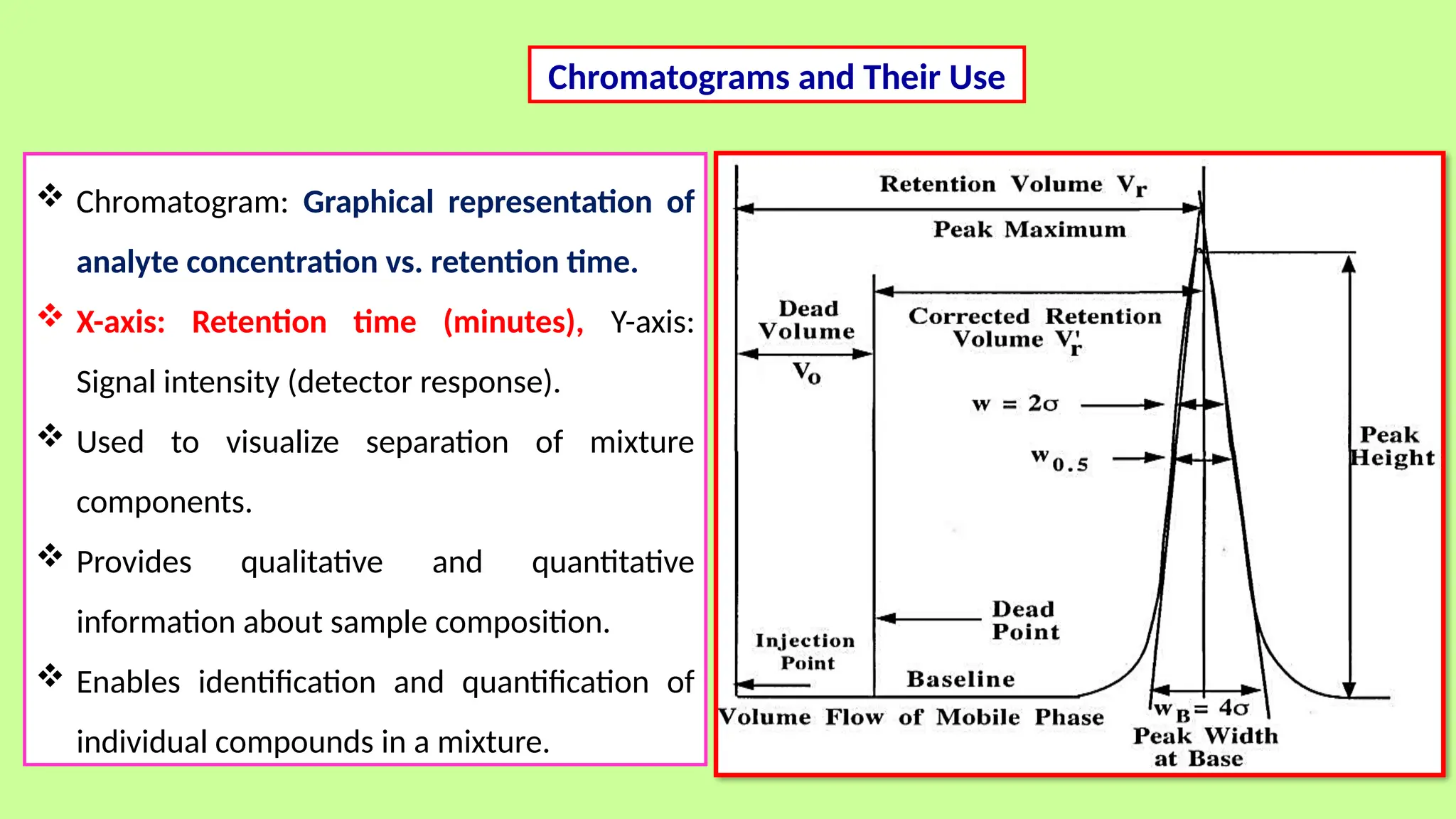
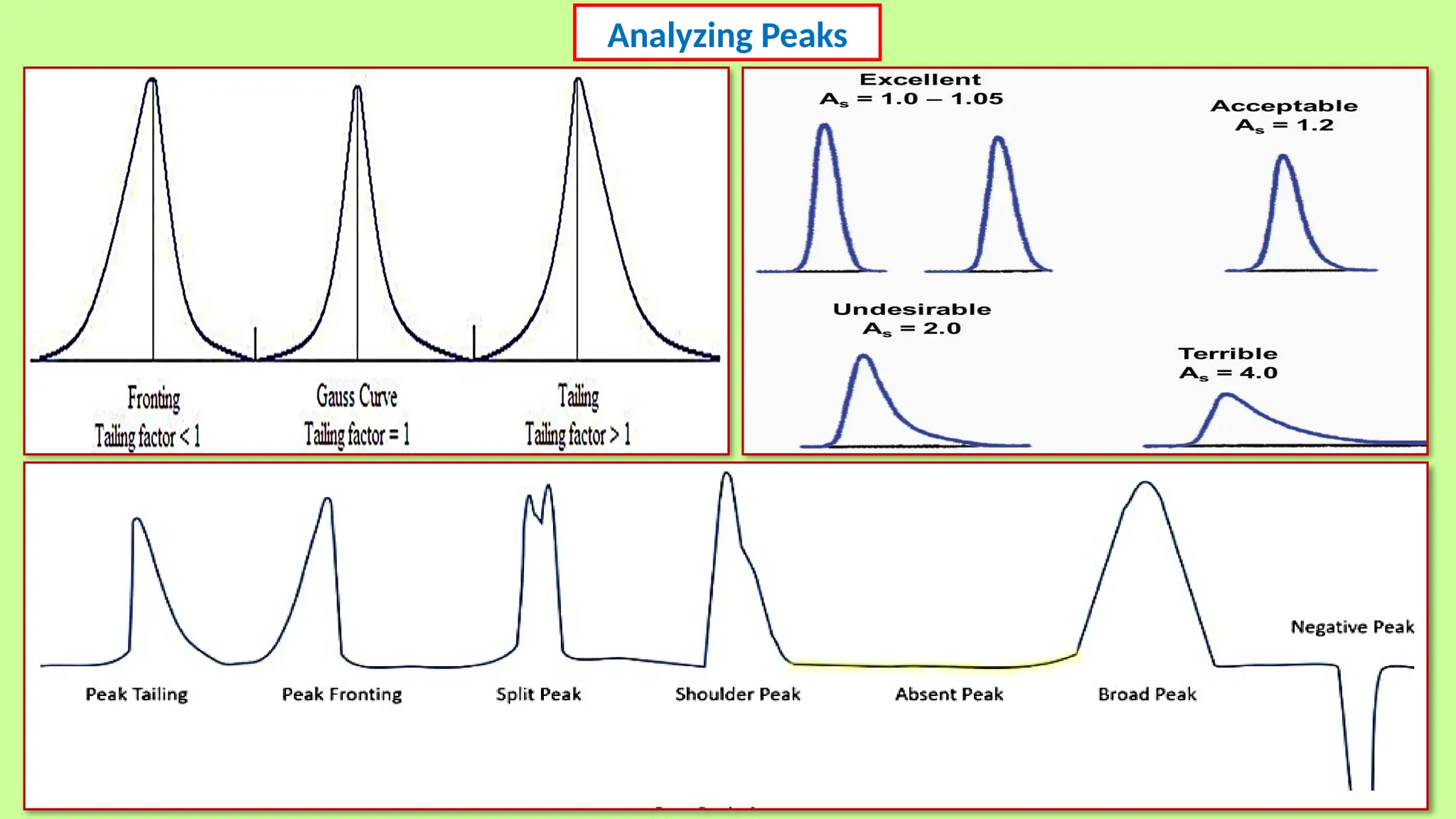
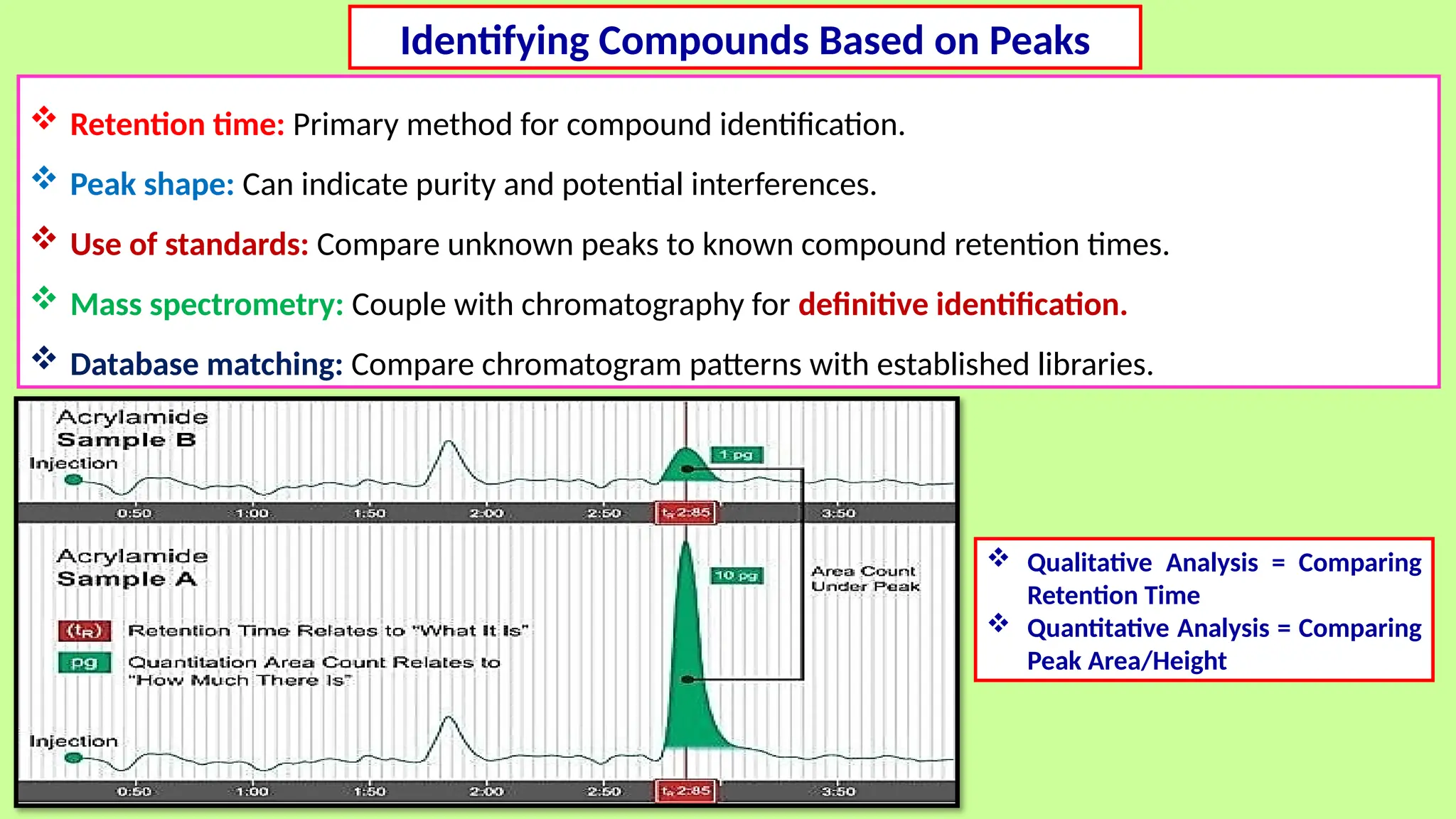
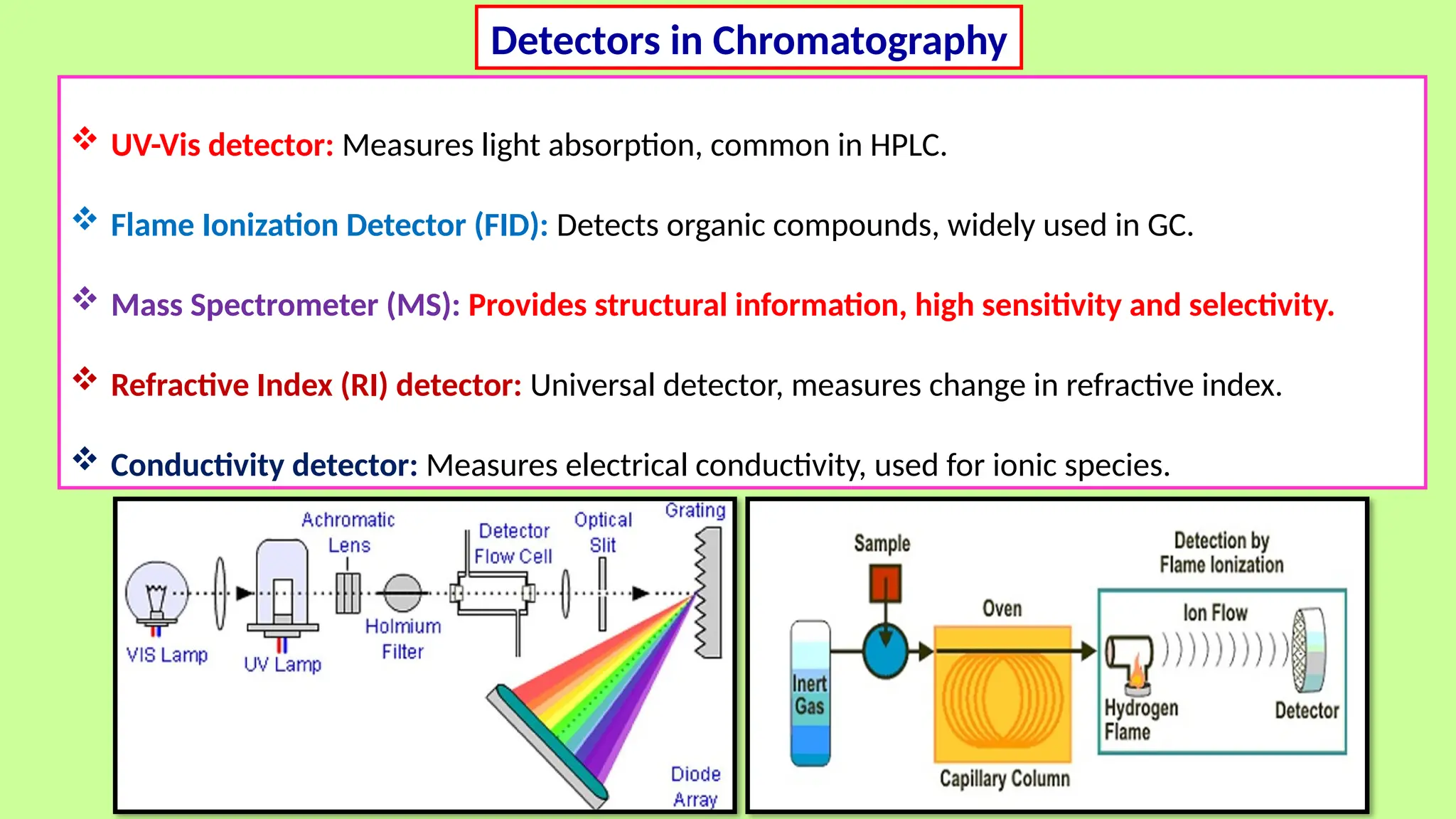
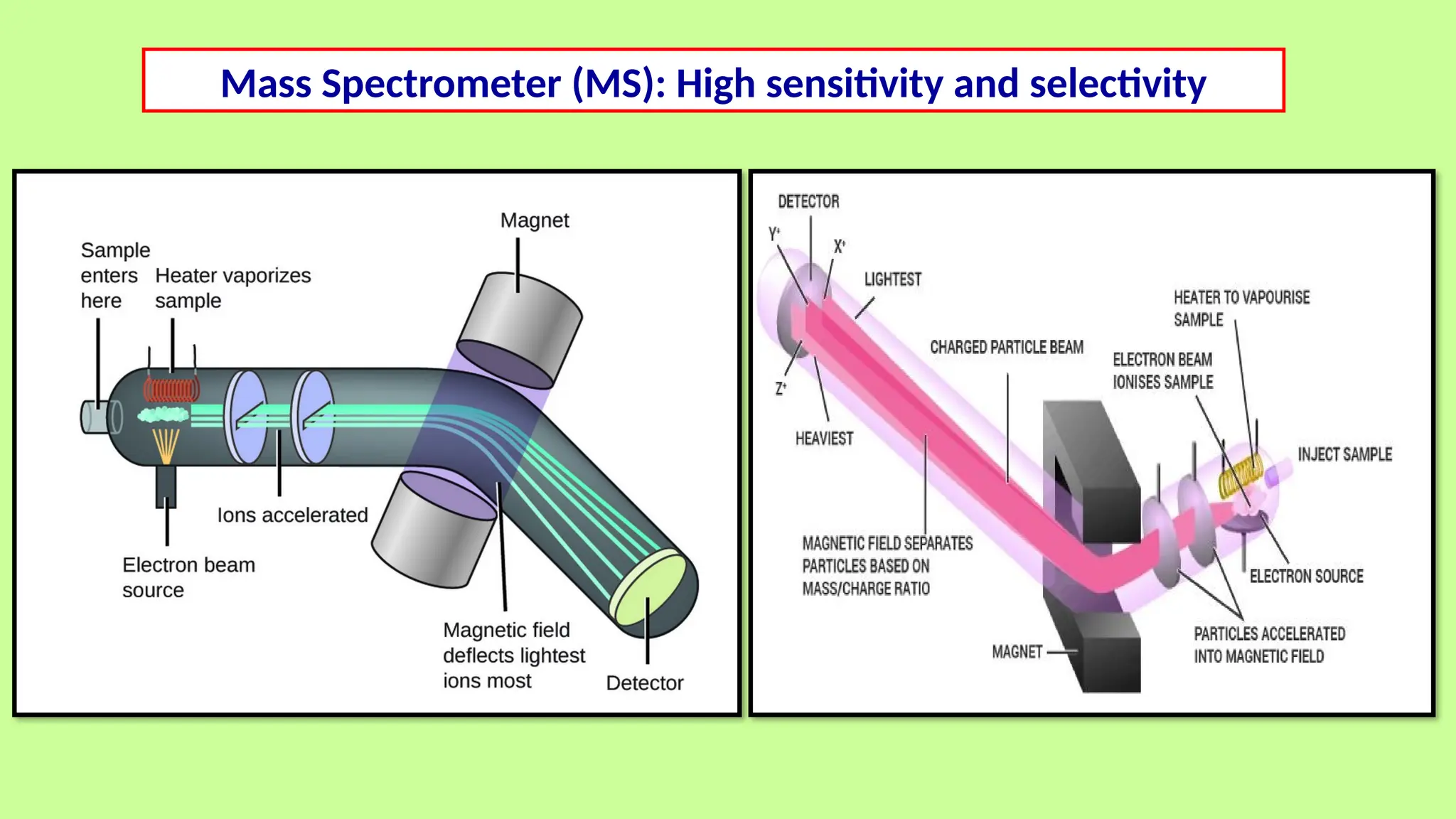
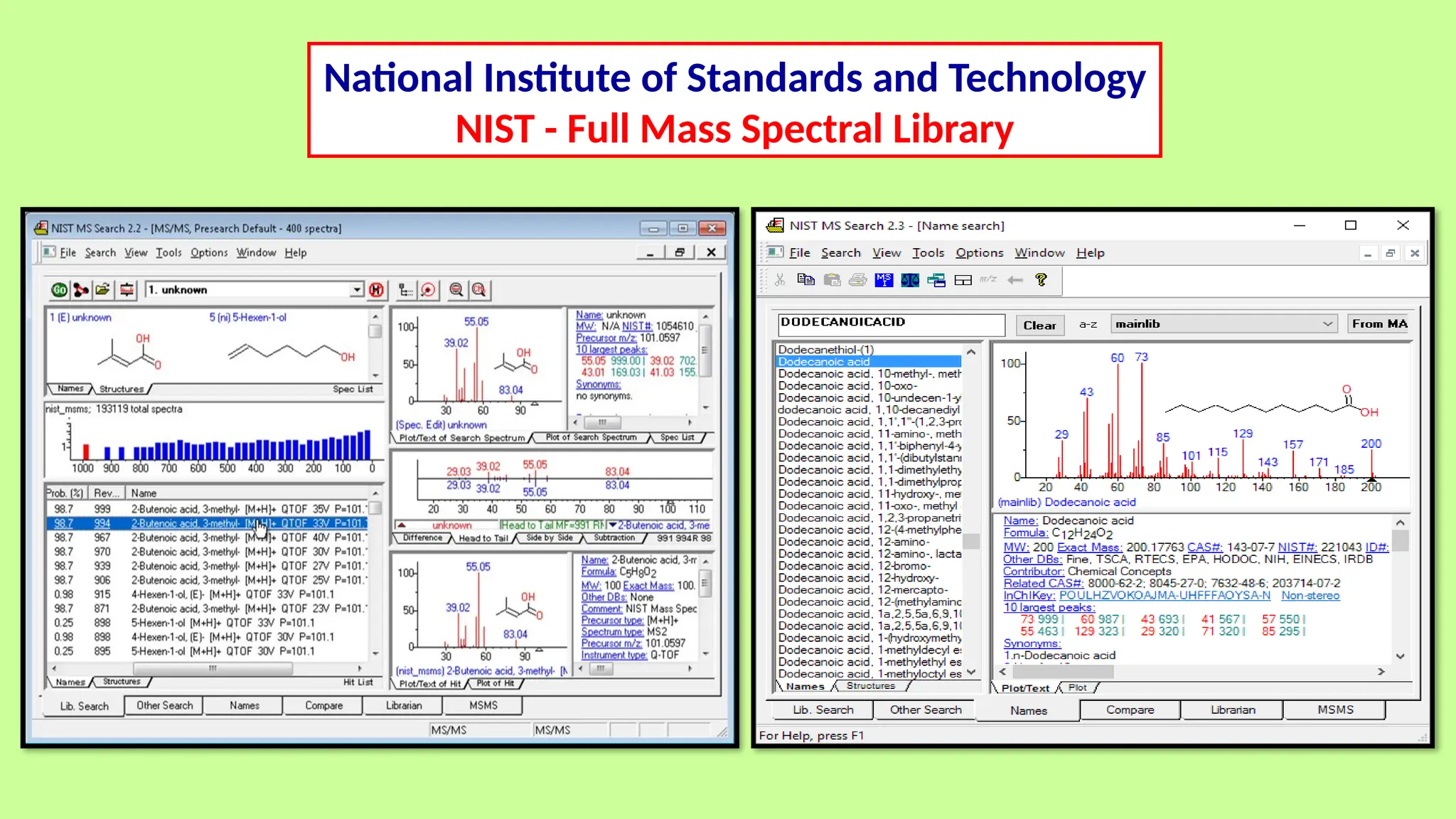
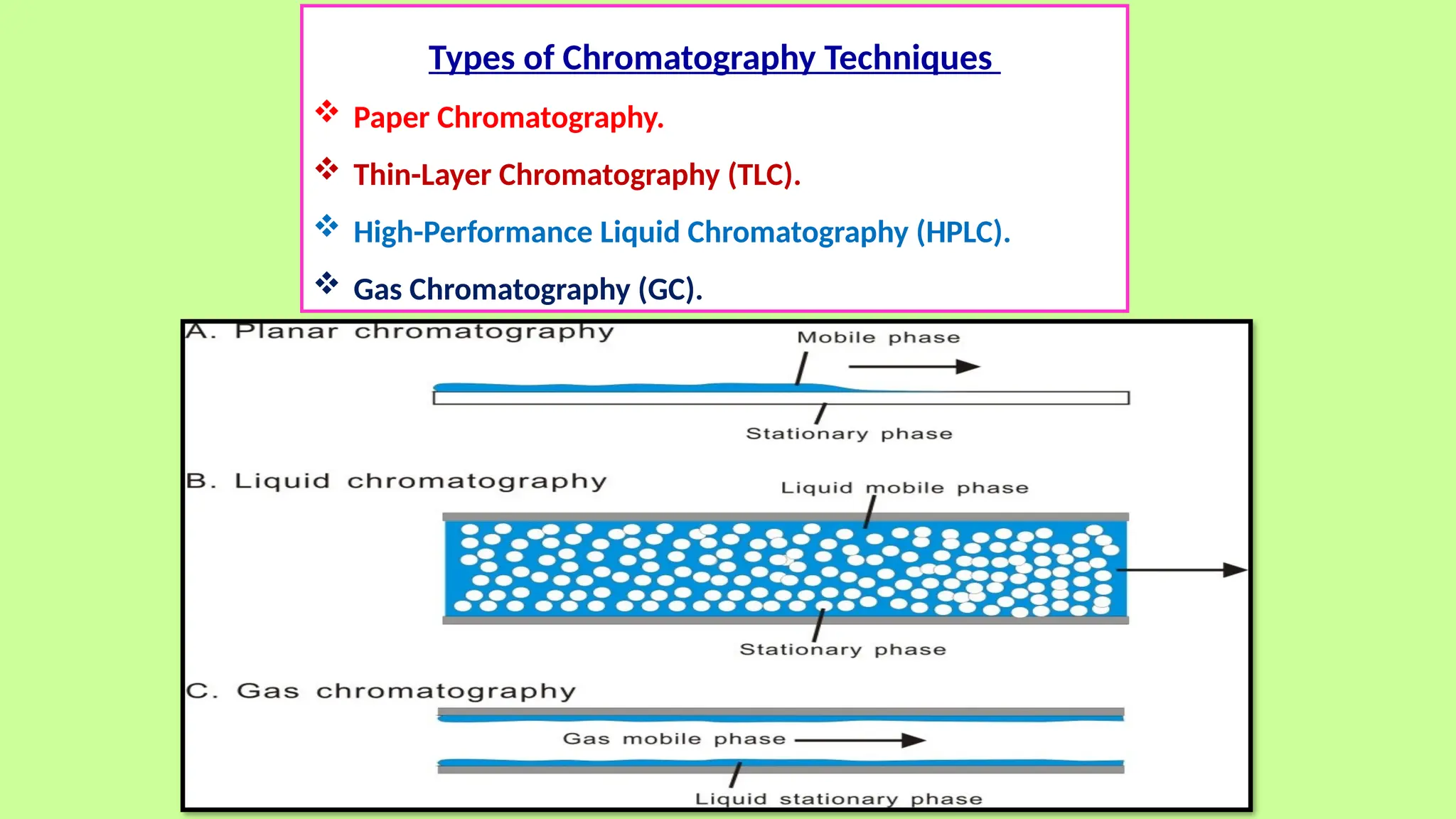
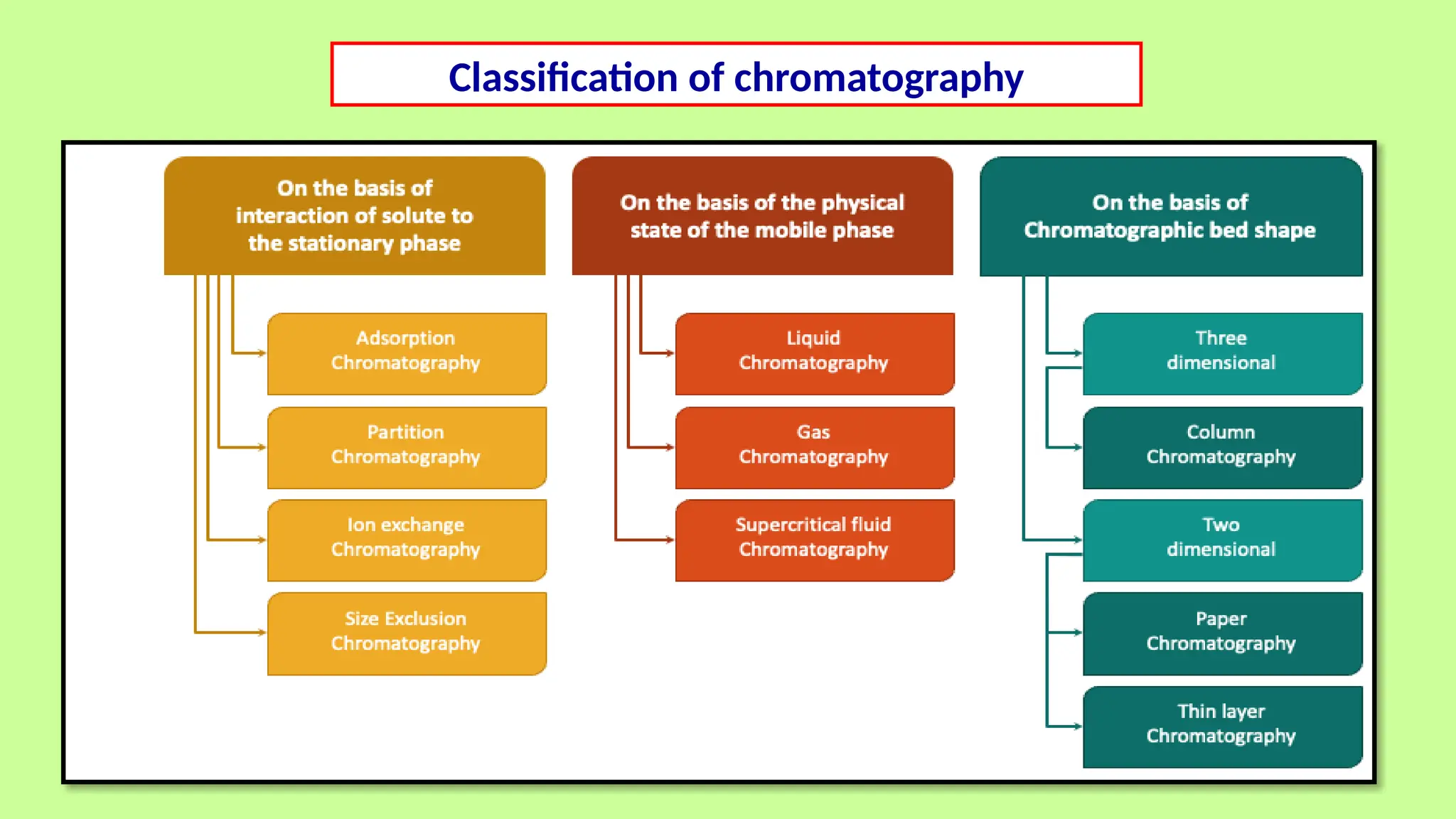
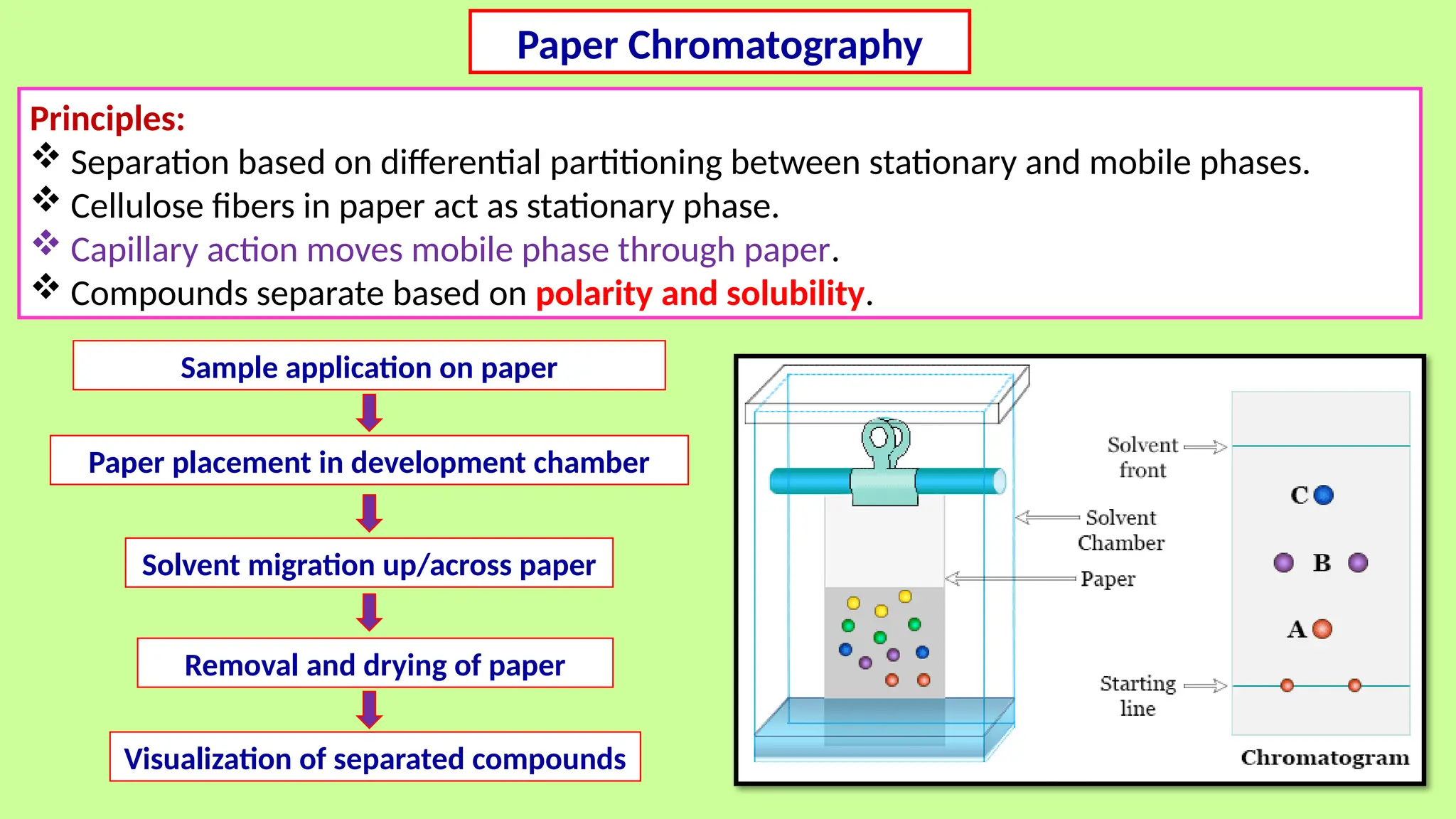
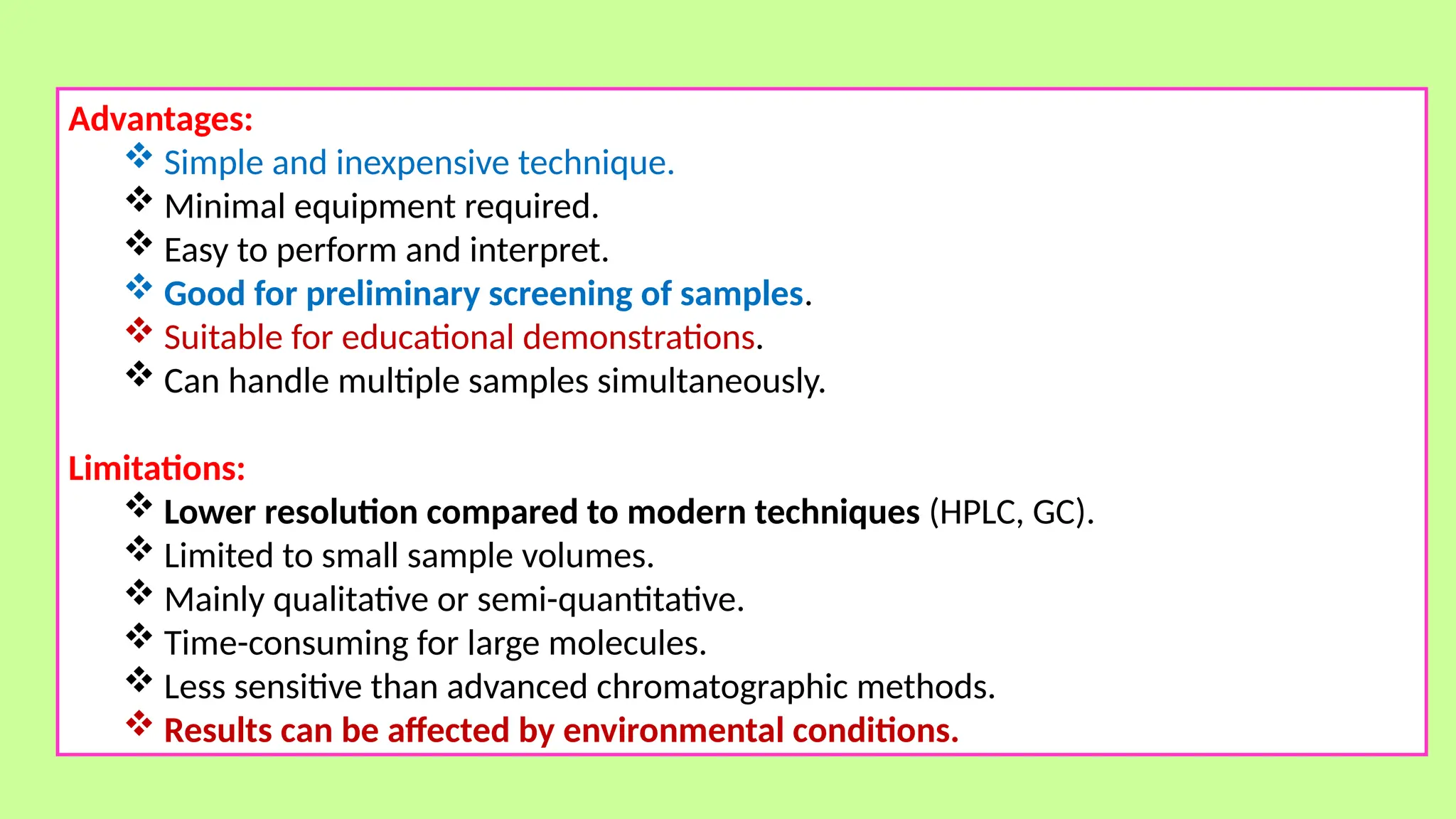
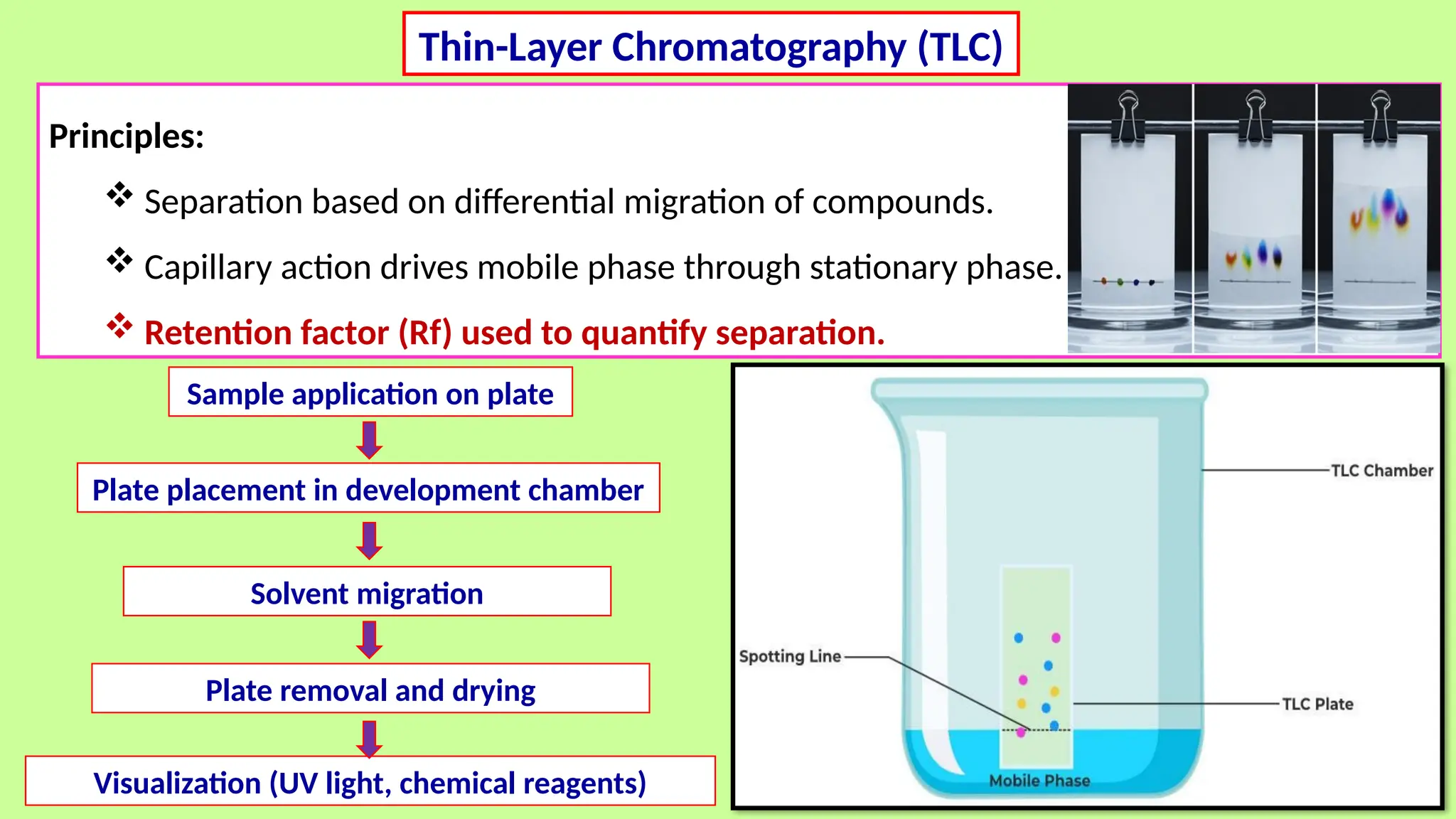
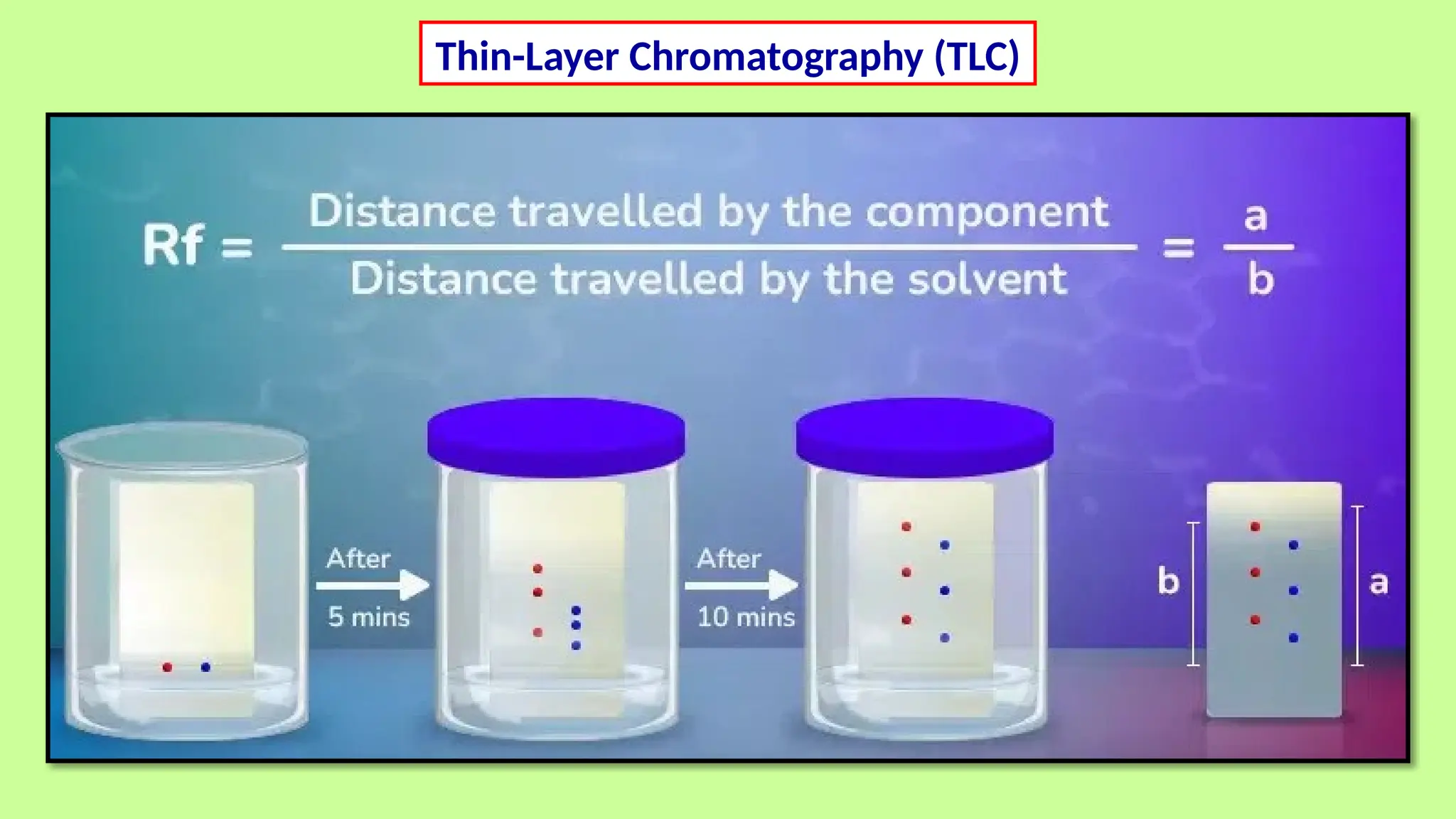
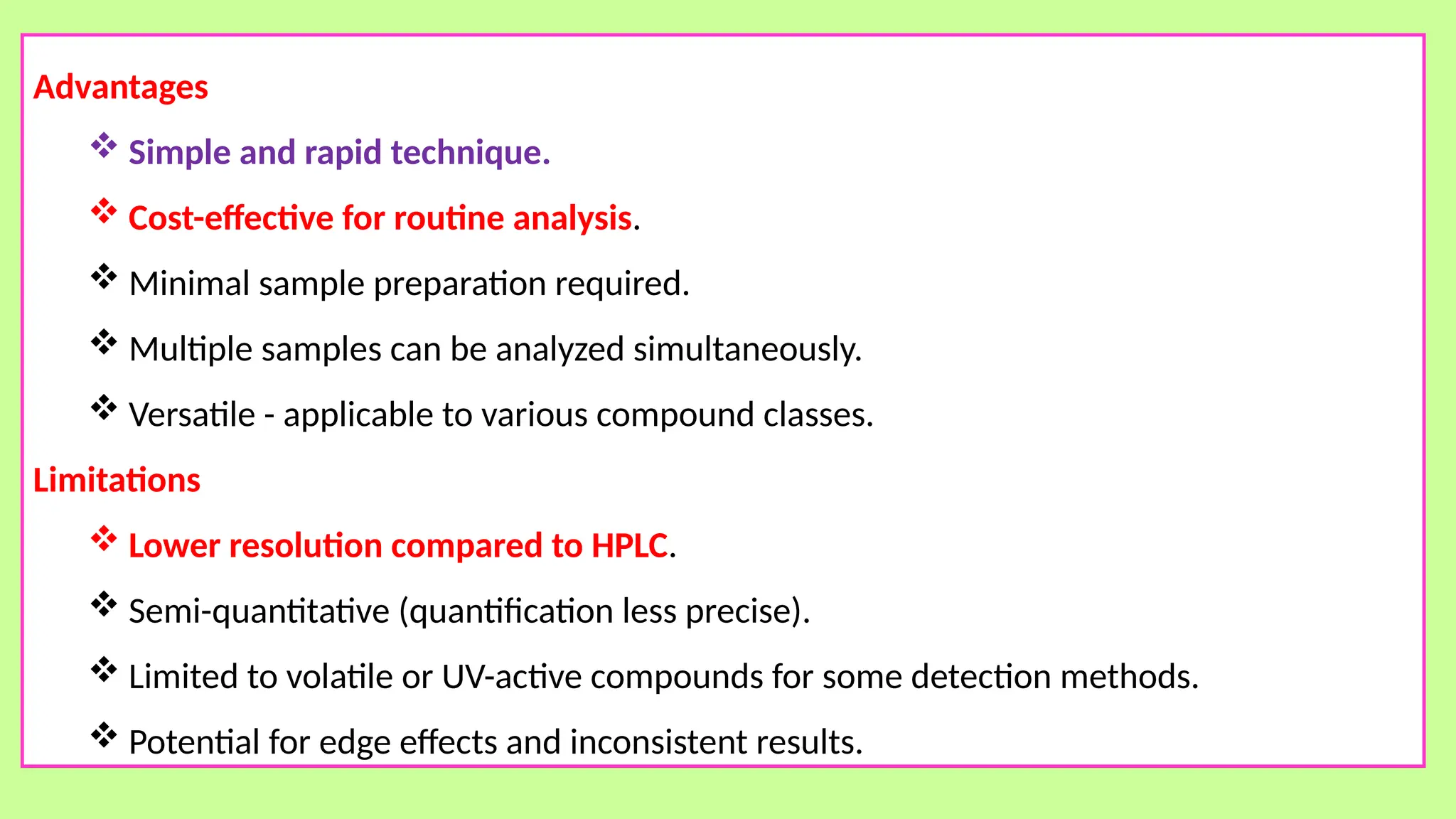
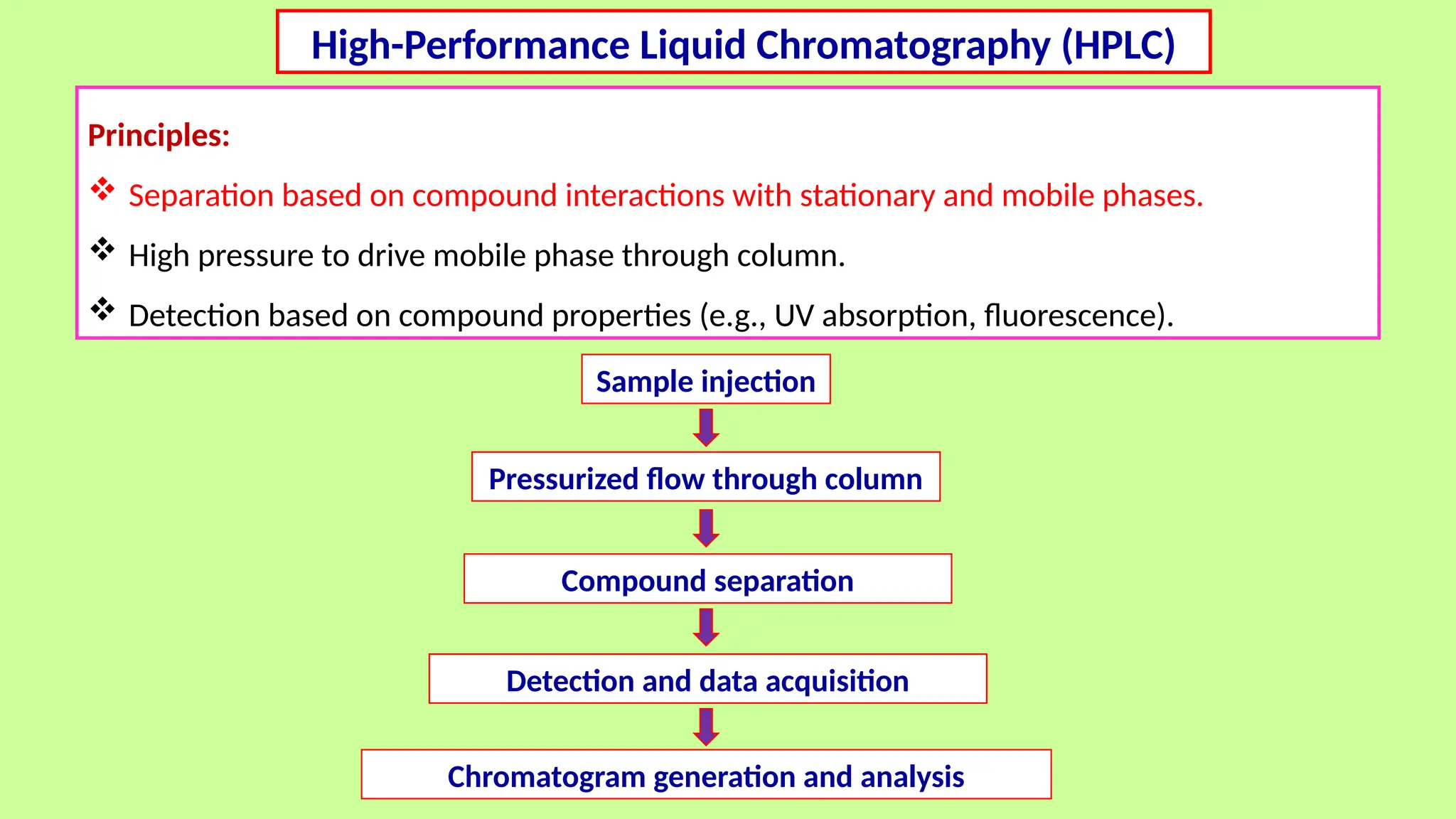
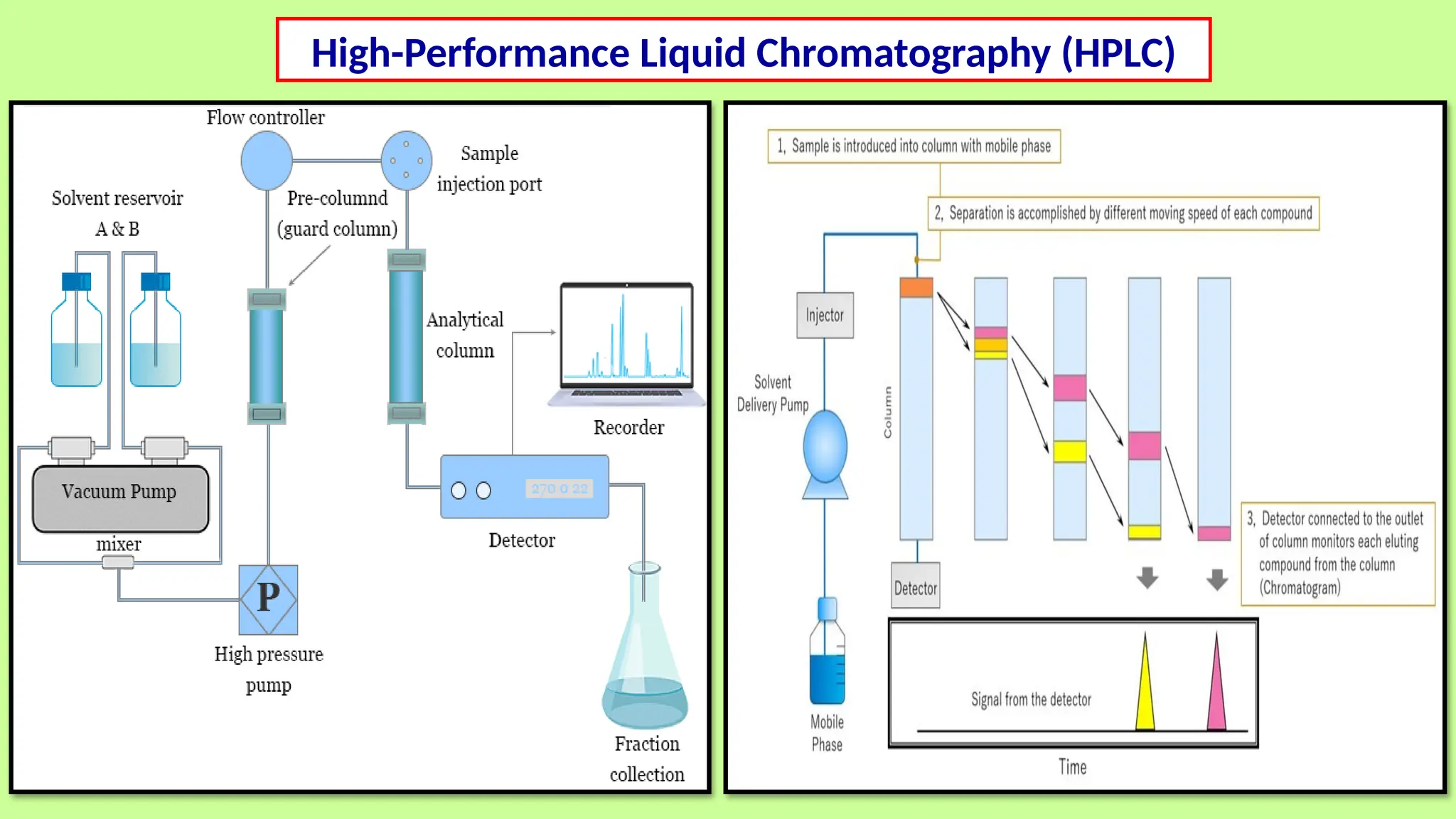
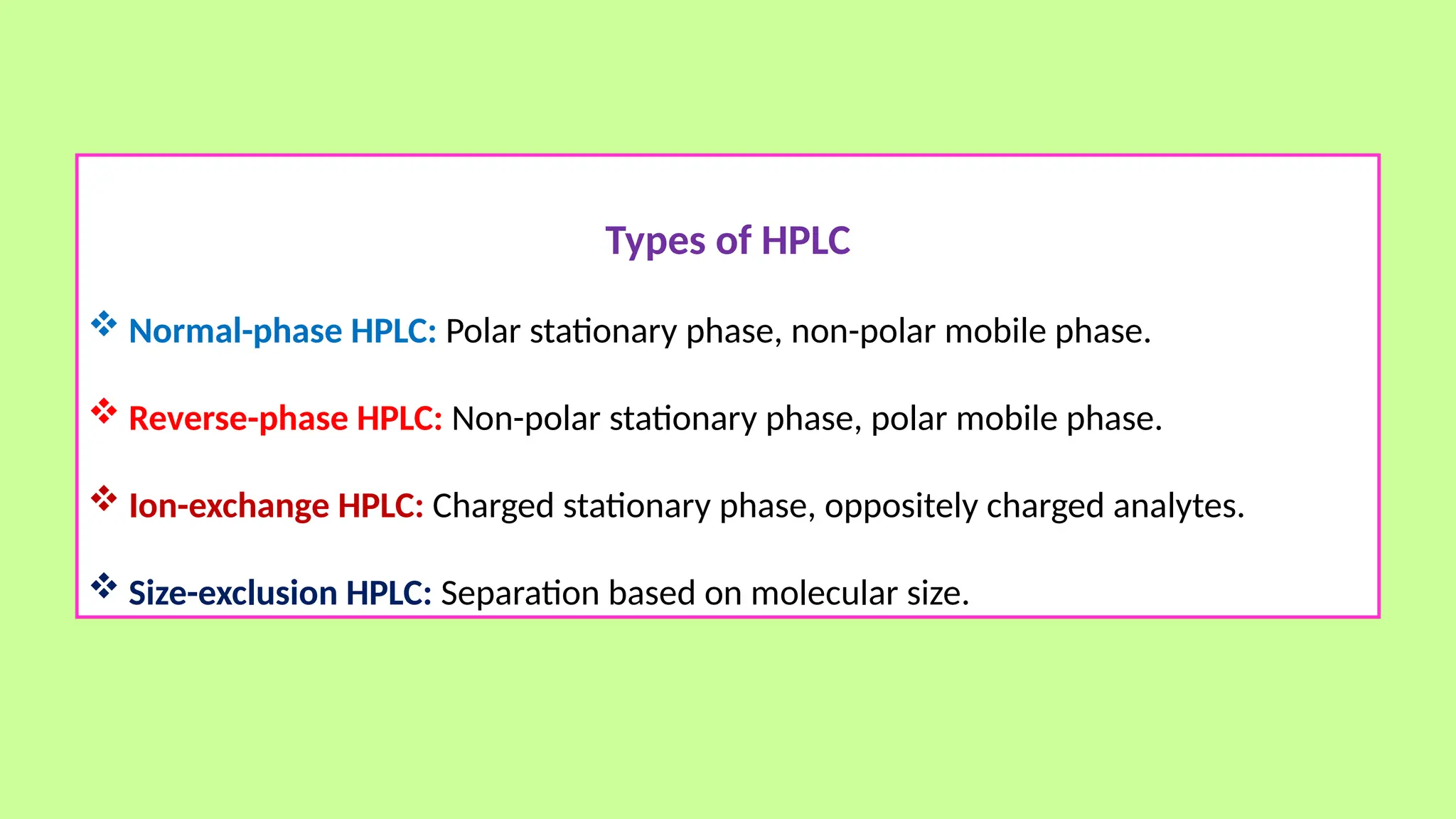
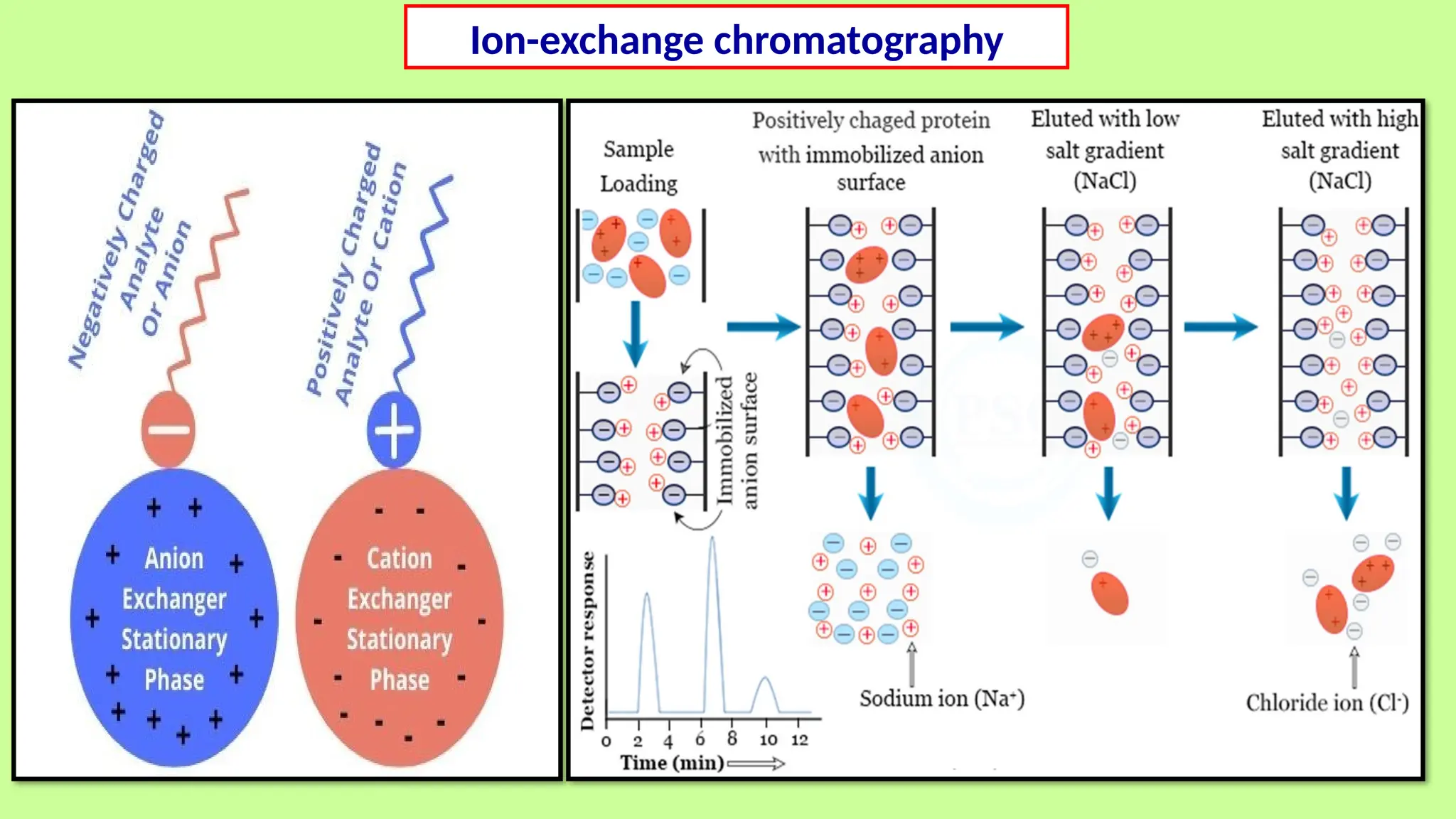
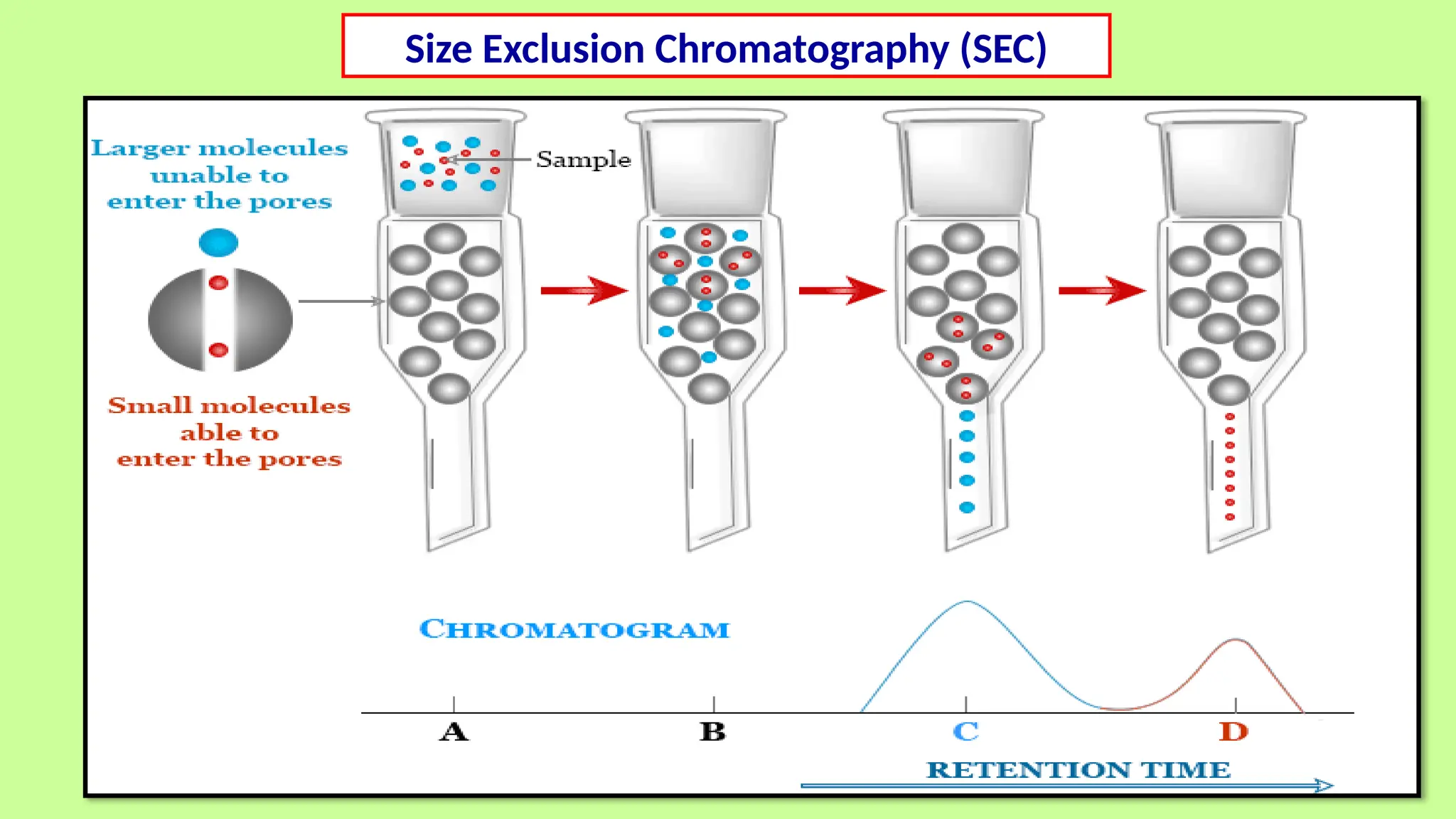
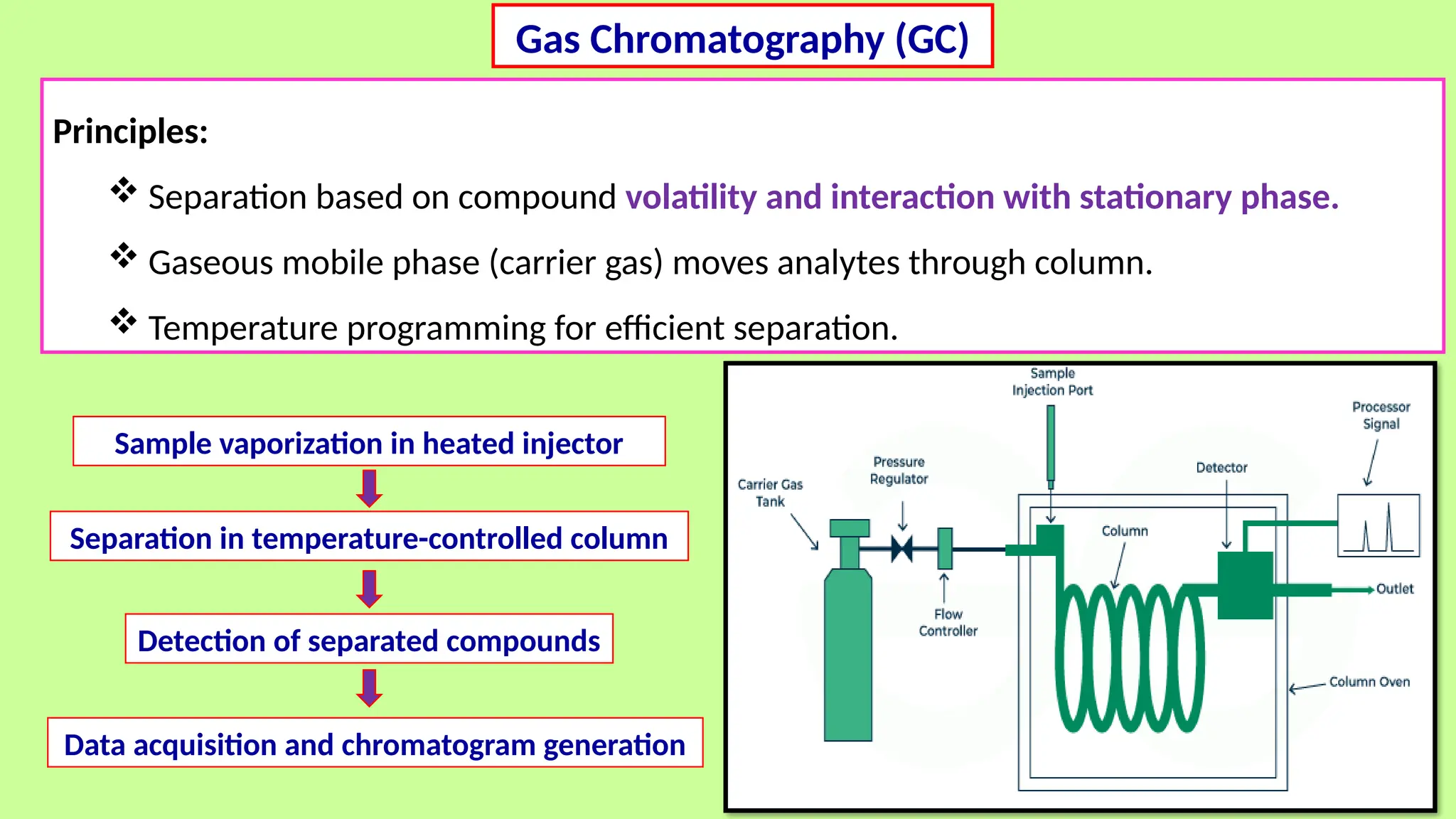
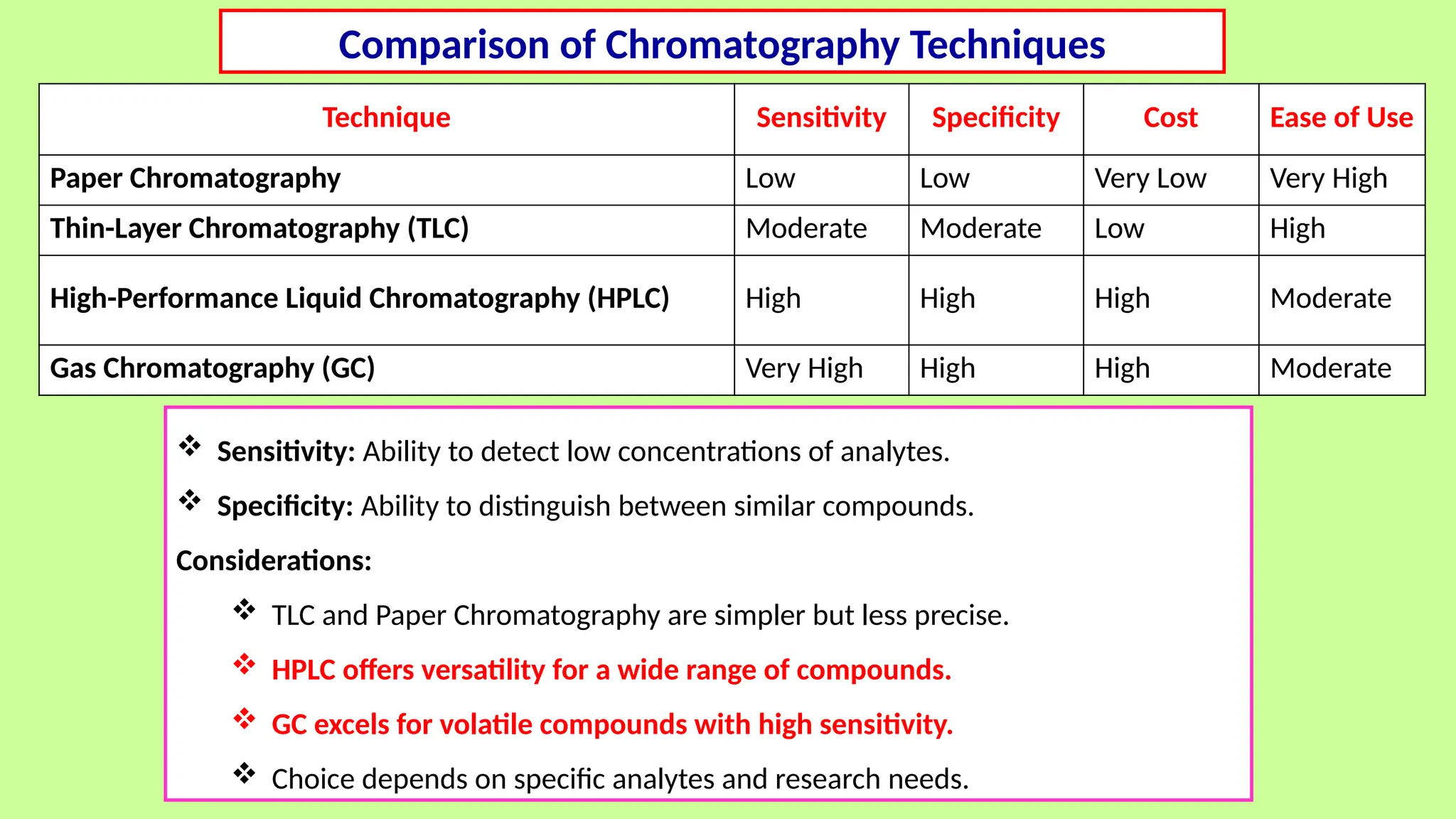
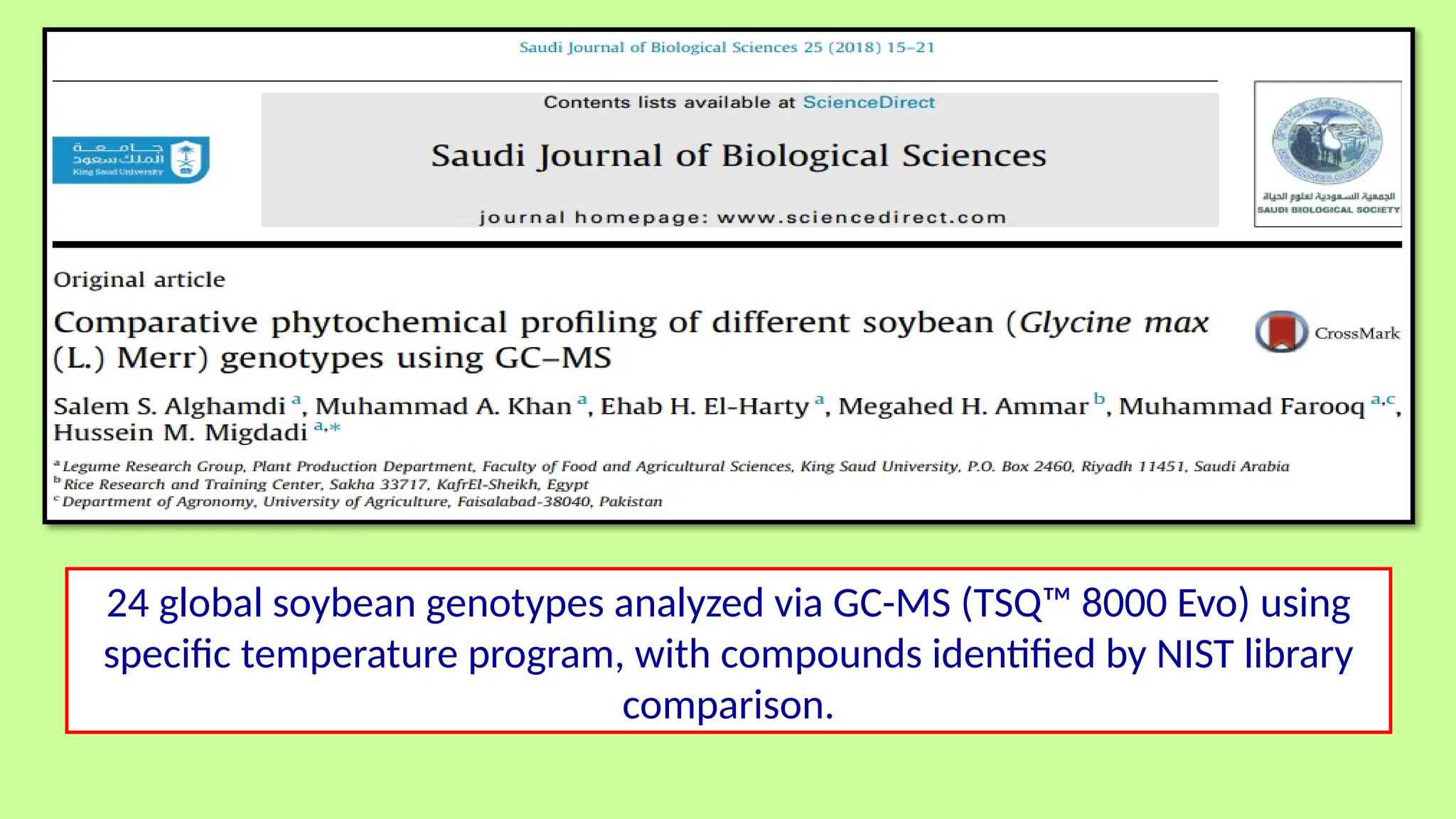
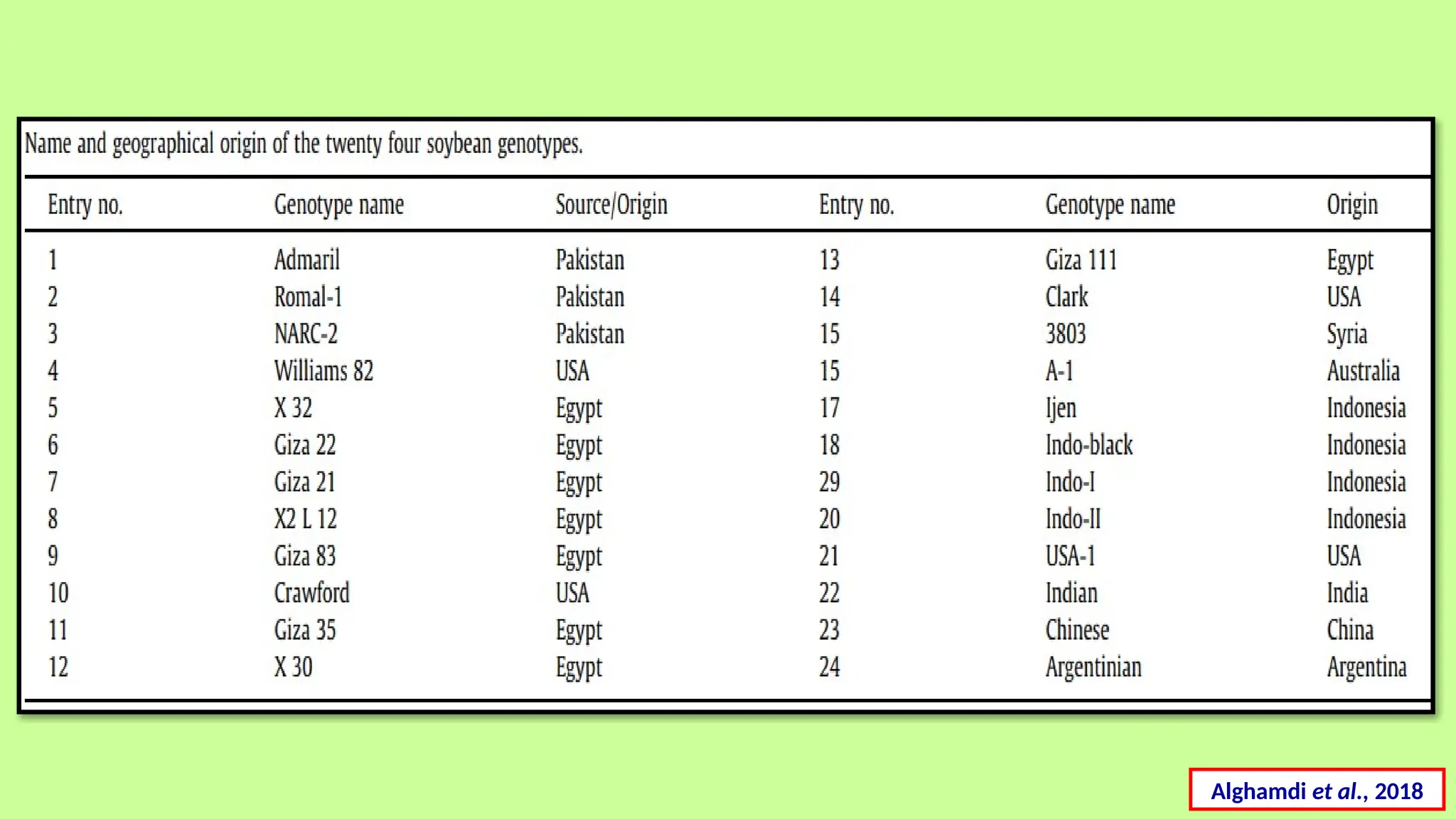
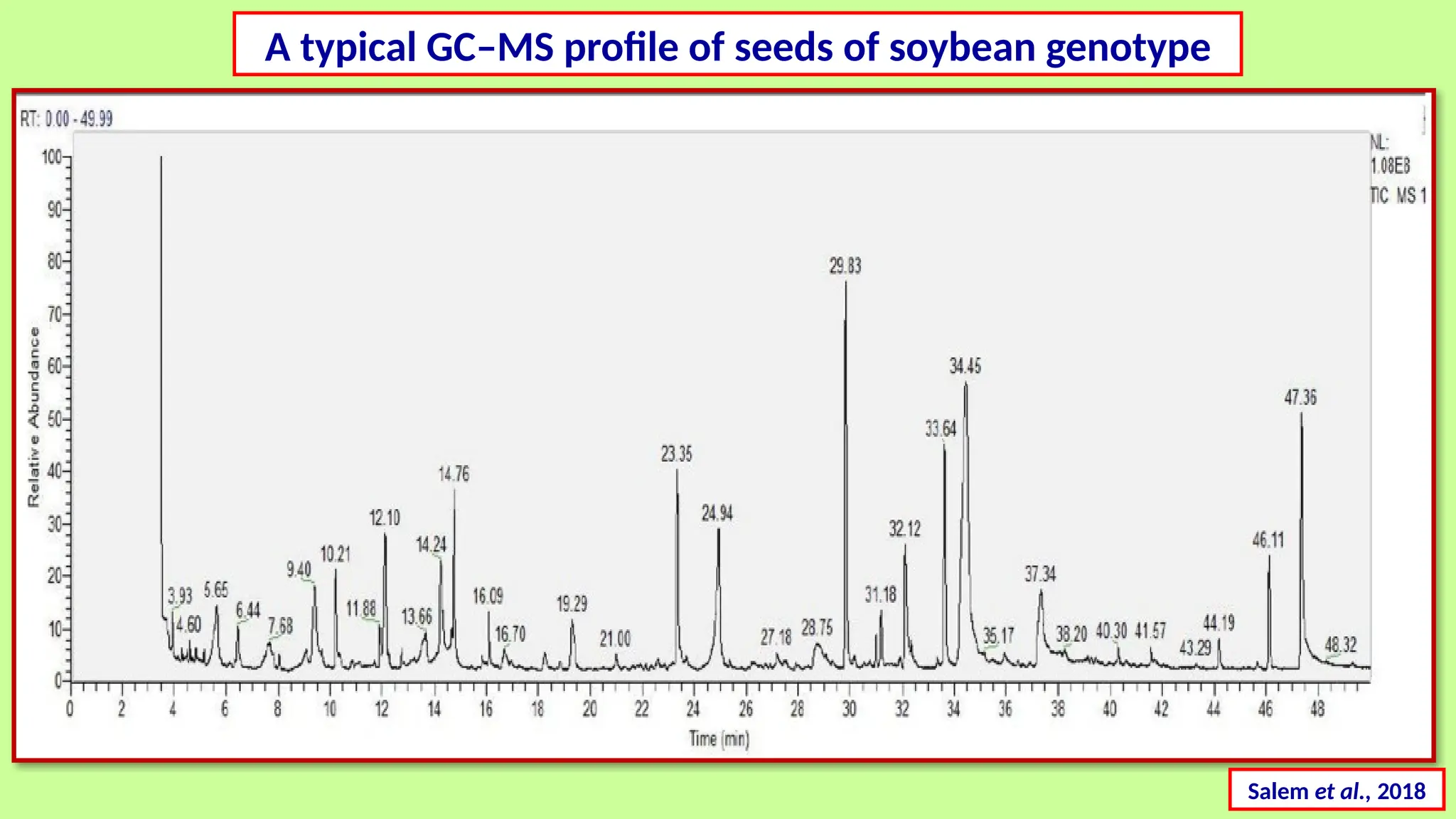
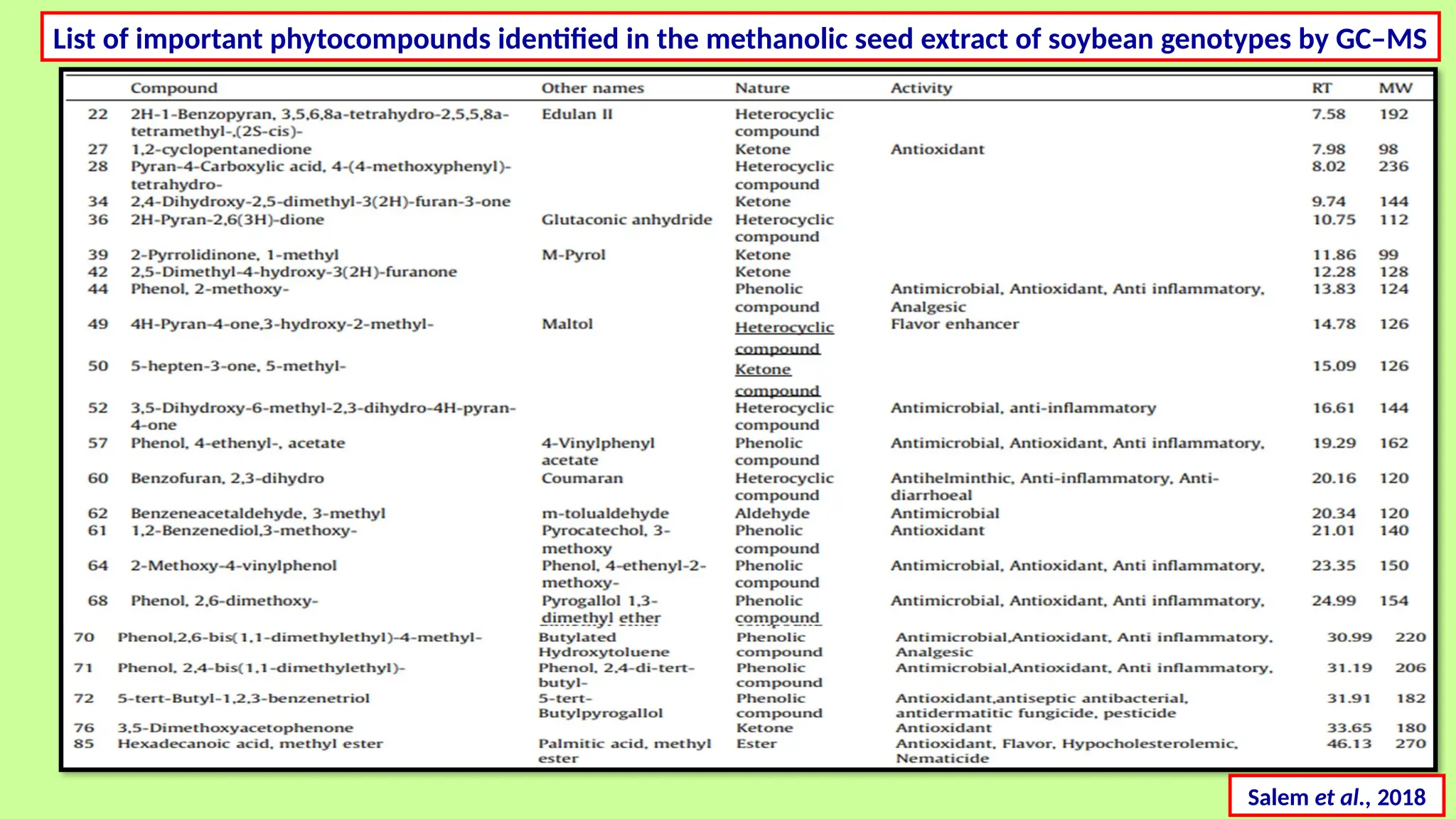
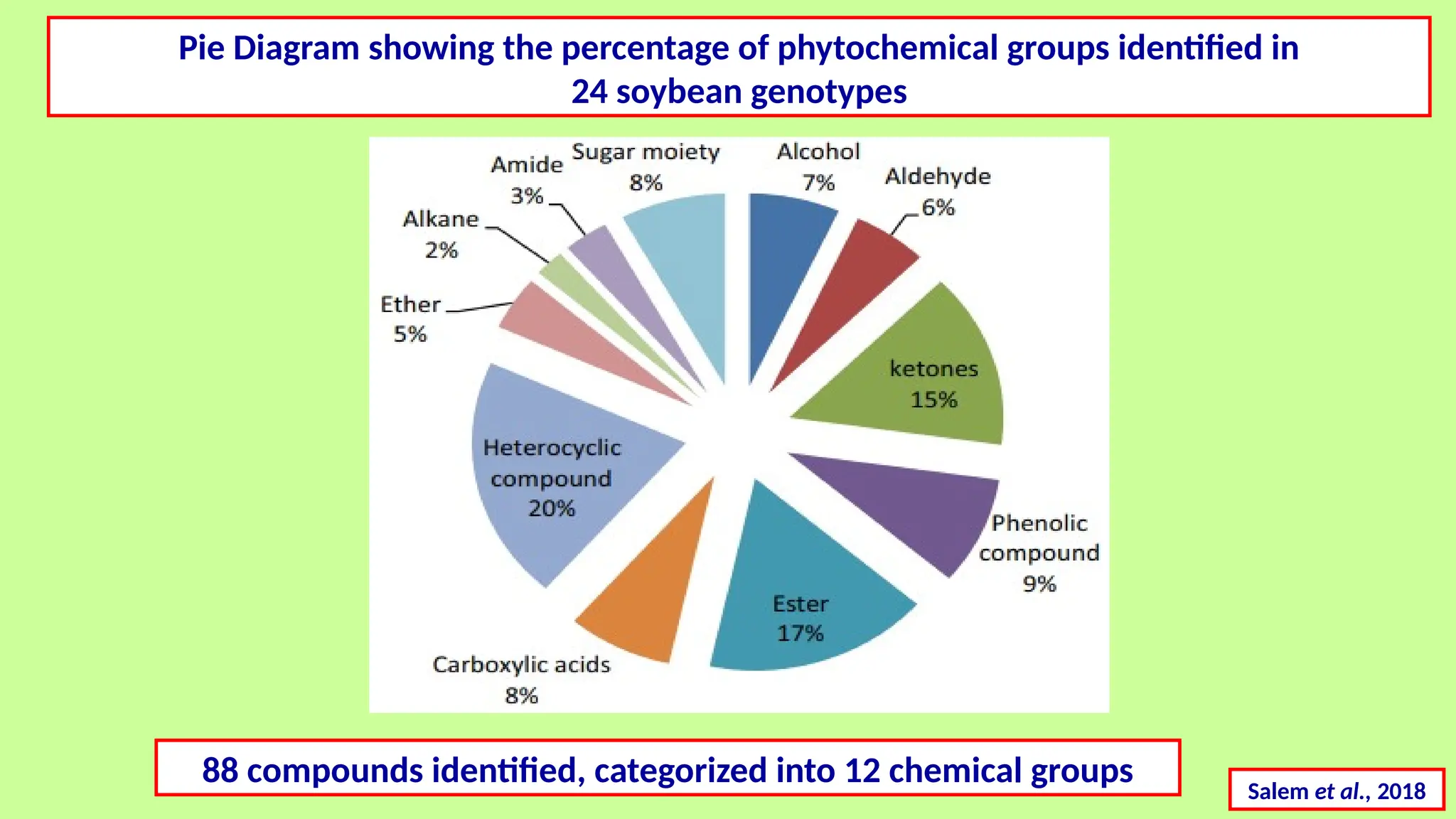
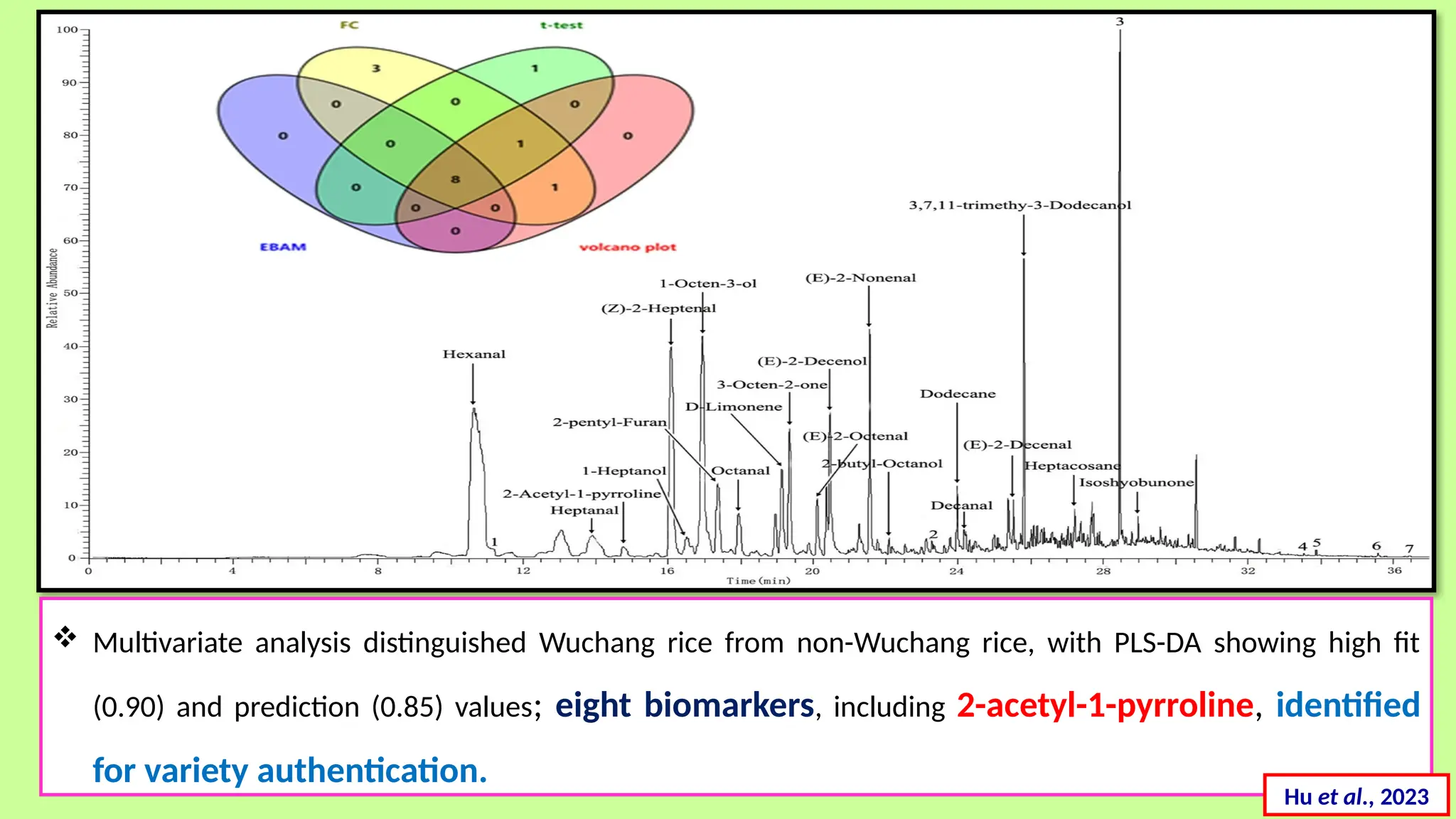
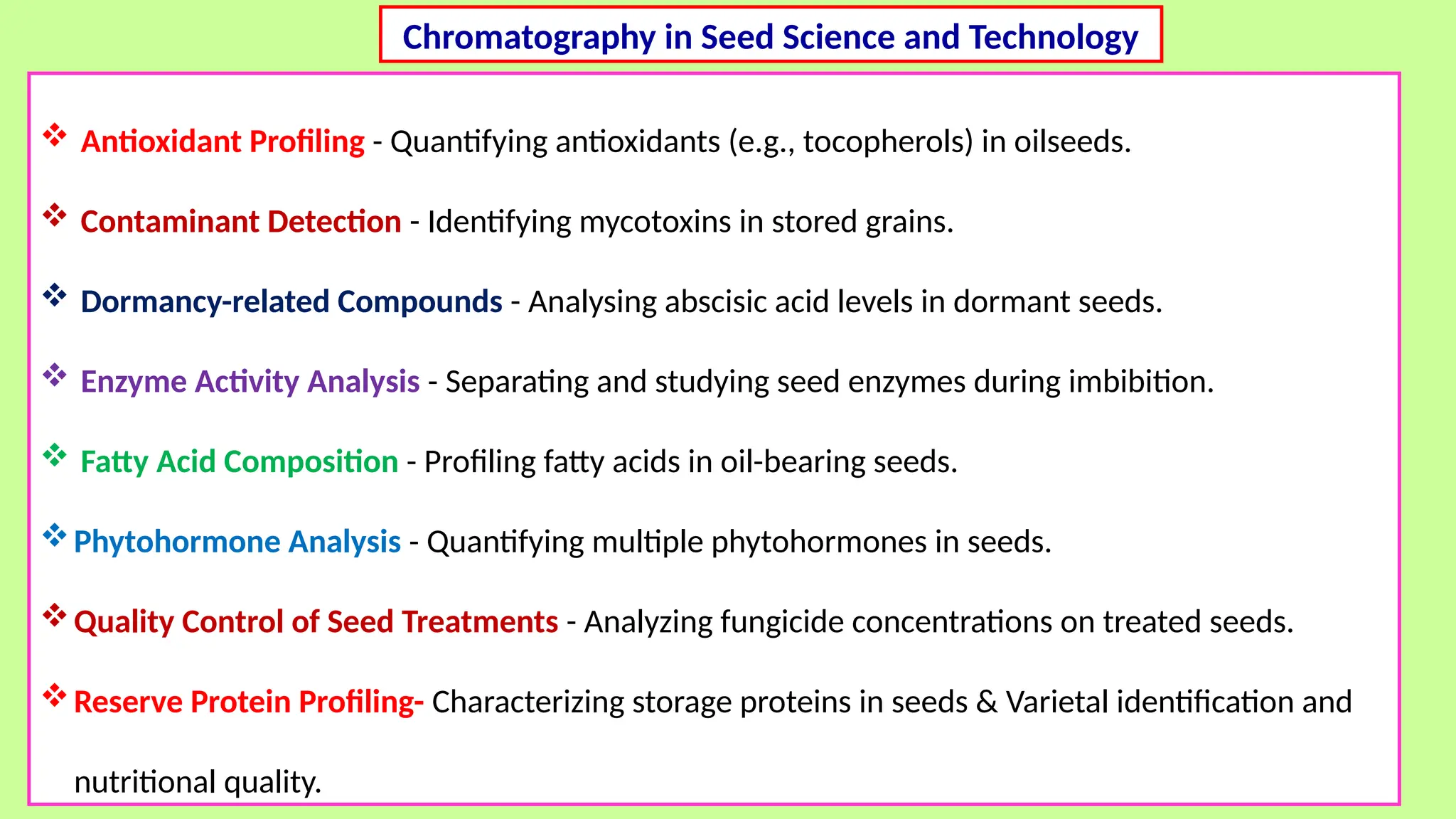
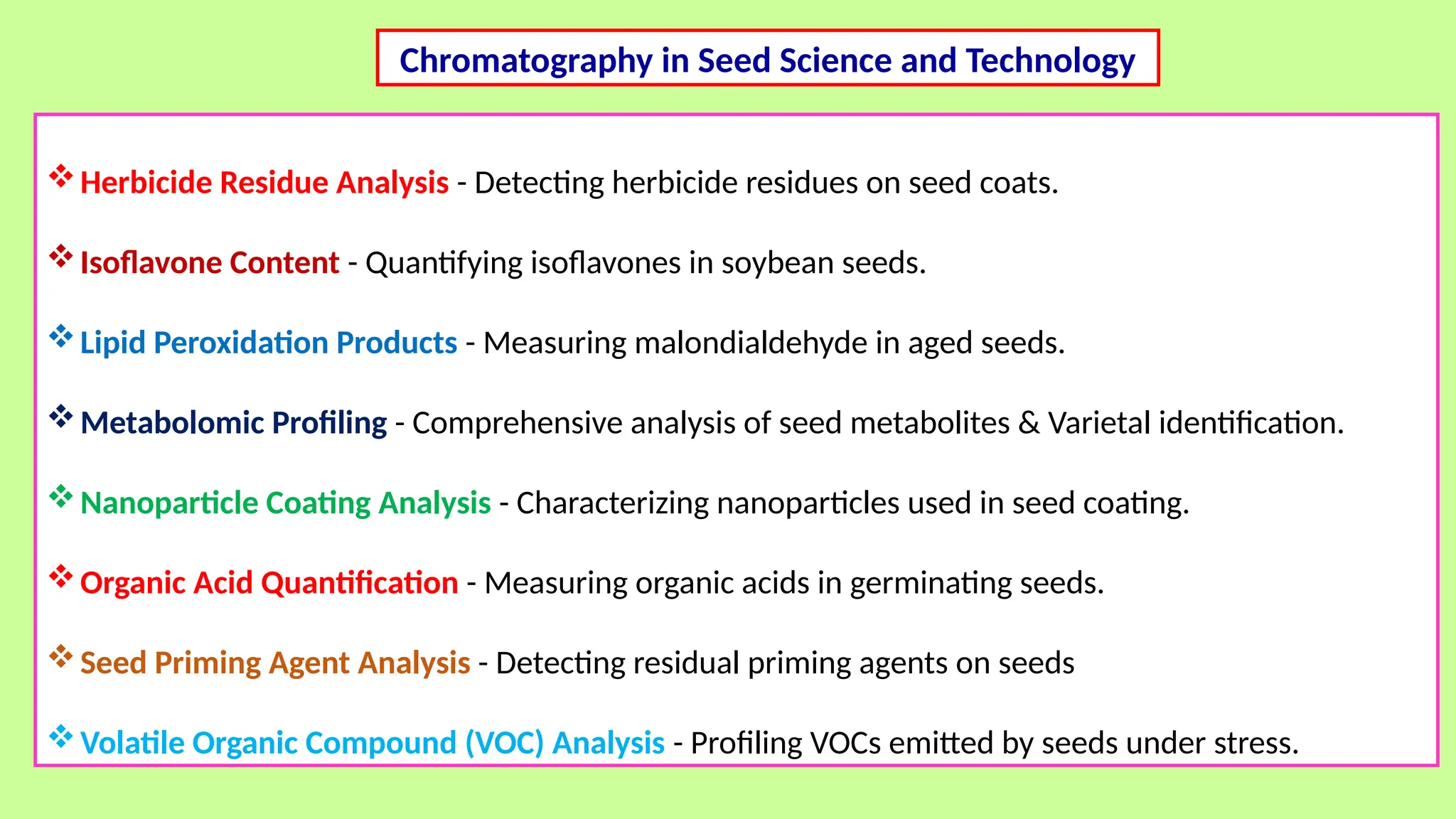
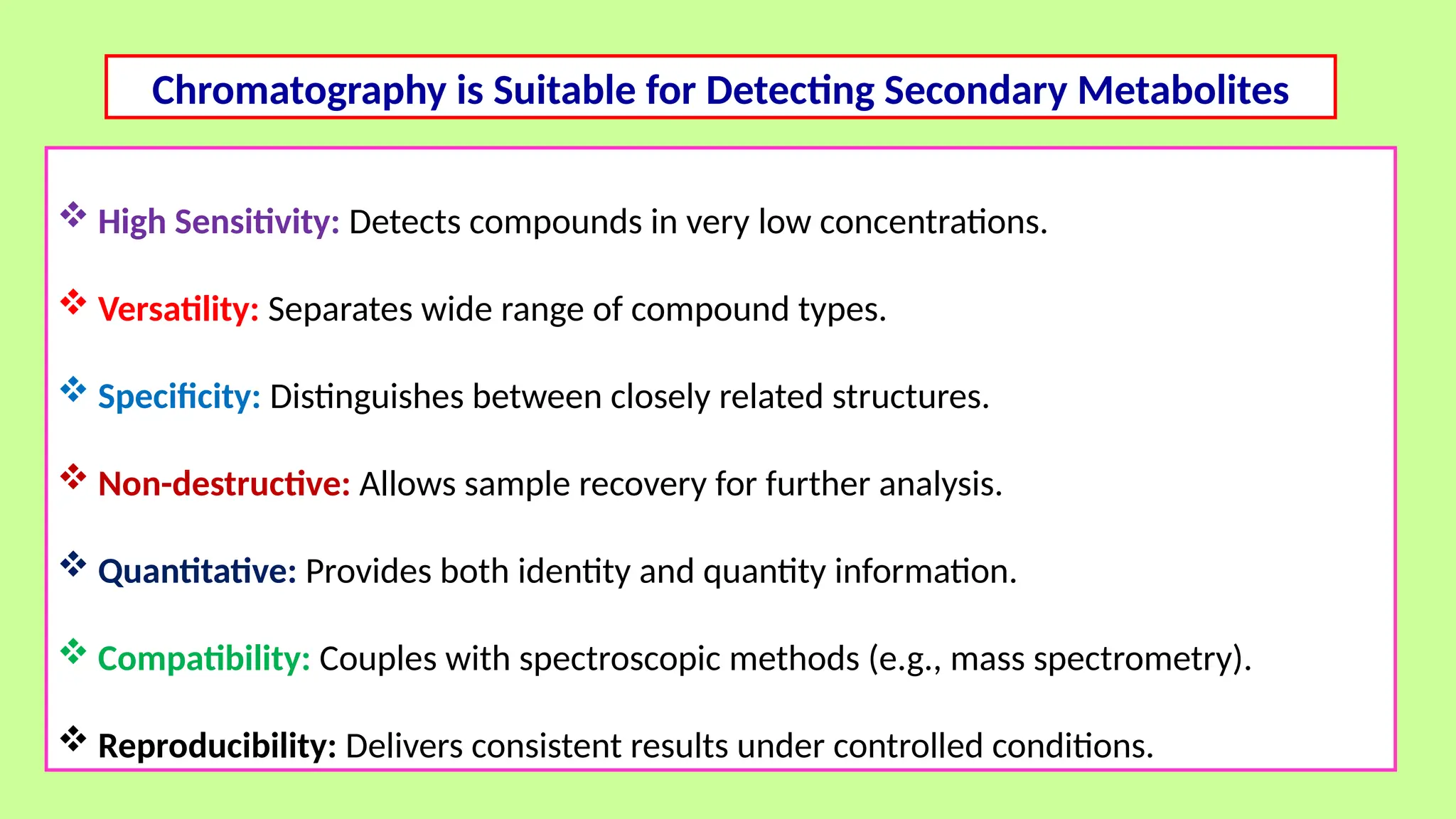
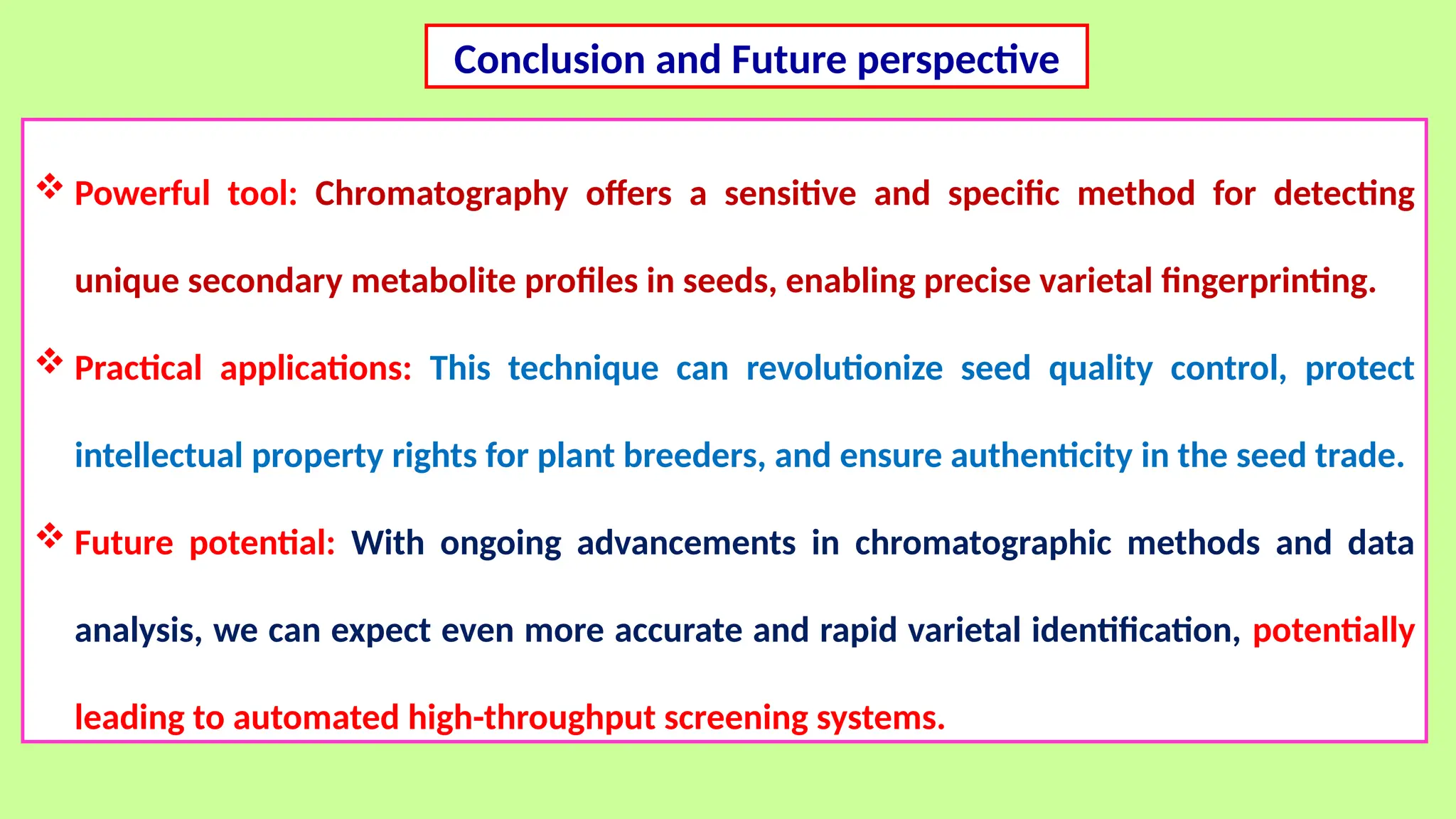
This comprehensive presentation explores the fundamental principles and applications of chromatography techniques in detecting secondary metabolites. Starting with the historical evolution of chromatography from Mikhail Tsvet's breakthrough in 1903 to modern advanced techniques, the presentation covers key chromatographic methods including Paper Chromatography, TLC, HPLC, and GC. It details the working principles, advantages, and limitations of each technique, supported by real-world case studies in seed science and agricultural research. Special emphasis is placed on the application of these techniques in analyzing secondary metabolites, particularly in seed science and technology. The presentation includes practical examples, comparative analyses, and future perspectives, making it valuable for students, researchers, and professionals in analytical chemistry, agricultural science, and biotechnology.