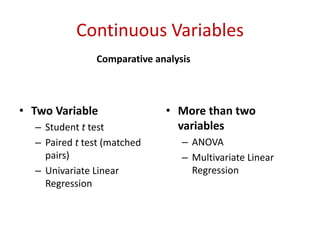
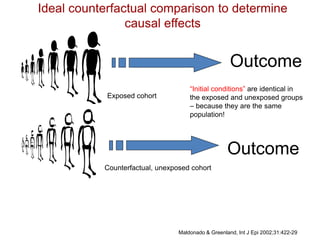
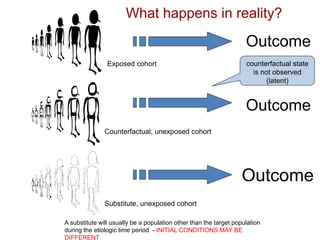
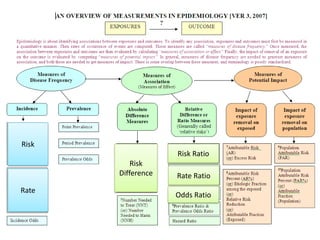
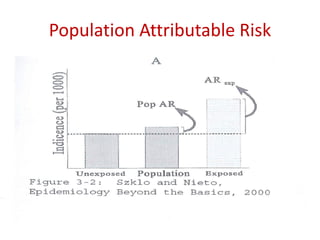
This document discusses choosing appropriate statistical tests based on study design and data type. It covers descriptive studies that measure prevalence and incidence, as well as analytic studies like randomized controlled trials, cohort studies, and case-control studies. For data type, it discusses approaches for continuous and categorical variables, including t-tests, ANOVA, chi-square tests, and regression. It also discusses measures of disease frequency, effect, and impact like risk difference, risk ratio, and odds ratio.

































![•Diagnostic 2 X 2 table*:
Disease + Disease -
Test + True
Positive
False
Positive
Test - False
Negative
True
Negative
*When test results are not dichotomous, then can use ROC curves [see later]
Diagnostic Studies](https://image.slidesharecdn.com/choosingappropriatestatisticaltest-140620072322-phpapp02/85/Choosing-appropriate-statistical-test-RSS6-2104-45-320.jpg)
![Disease
present
Disease
absent
Test
positive
True
positives
False
positives
Test
negative
False
negative
True
negatives
Sensitivity
[true positive rate]
The proportion of patients with disease who test
positive = P(T+|D+) = TP / (TP+FN)](https://image.slidesharecdn.com/choosingappropriatestatisticaltest-140620072322-phpapp02/85/Choosing-appropriate-statistical-test-RSS6-2104-46-320.jpg)
![Disease
present
Disease
absent
Test
positive
True
positives
False
positives
Test
negative
False
negative
True
negatives
Specificity
[true negative rate]
The proportion of patients without disease who test
negative: P(T-|D-) = TN / (TN + FP).](https://image.slidesharecdn.com/choosingappropriatestatisticaltest-140620072322-phpapp02/85/Choosing-appropriate-statistical-test-RSS6-2104-47-320.jpg)