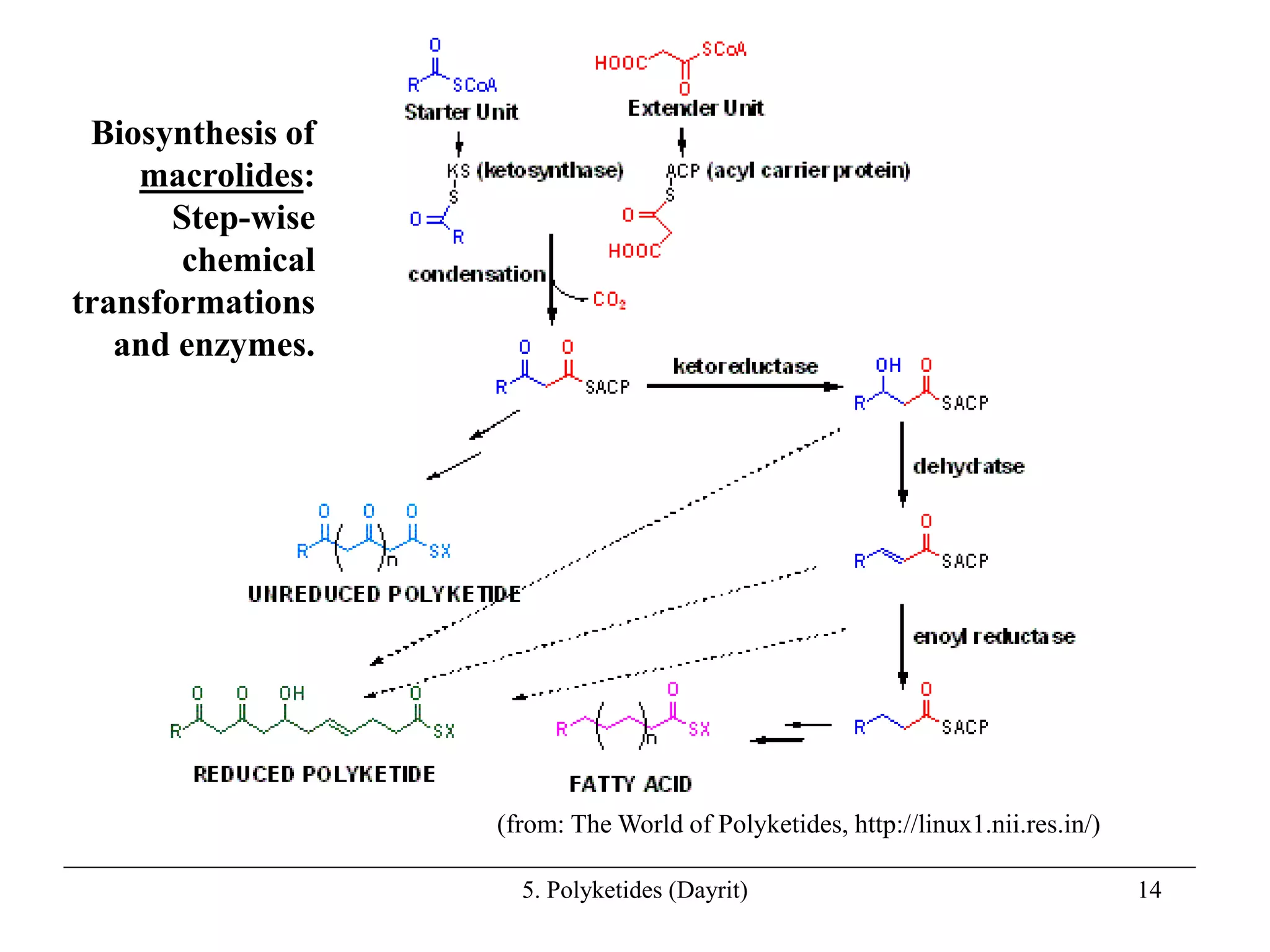
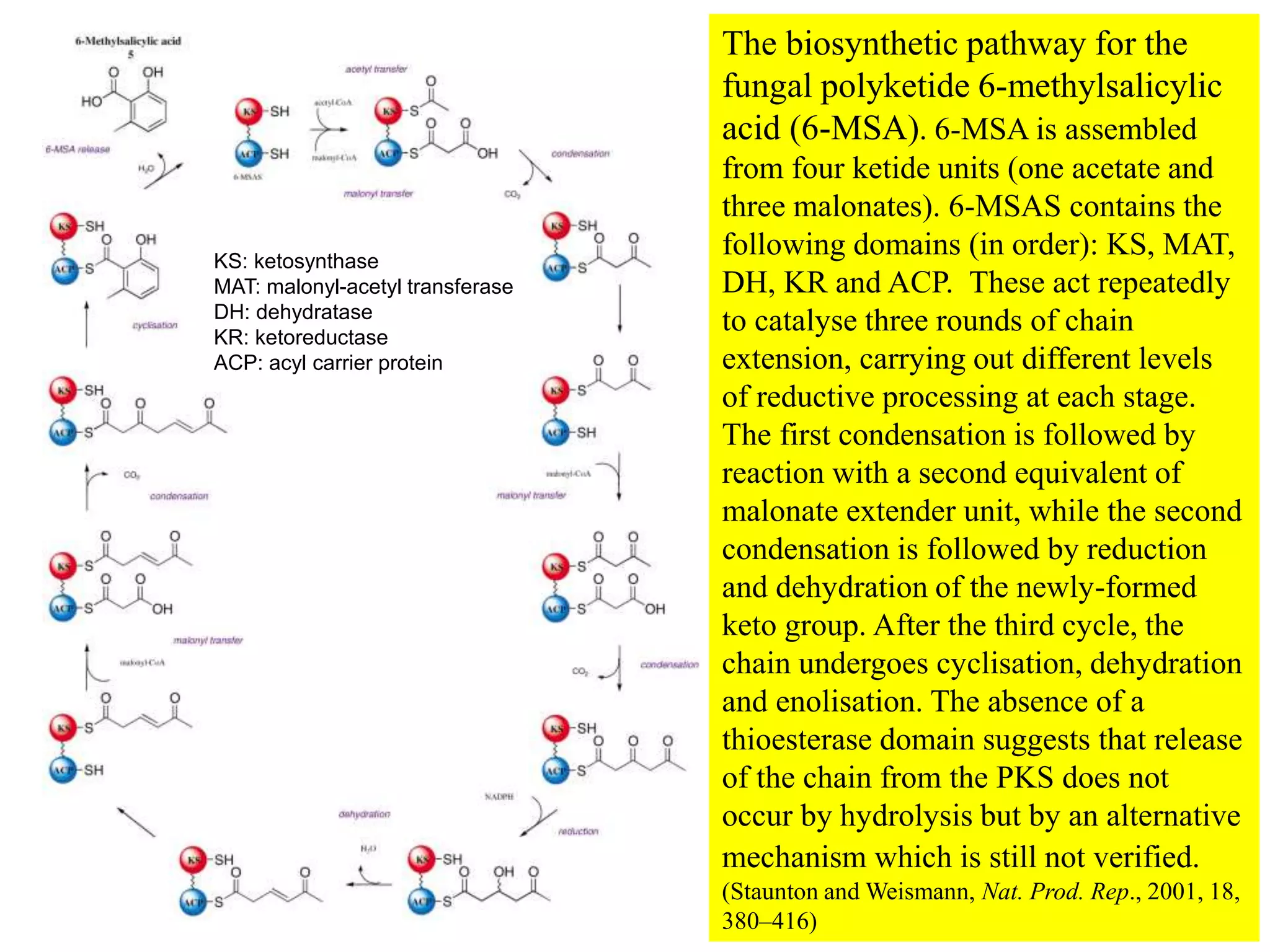
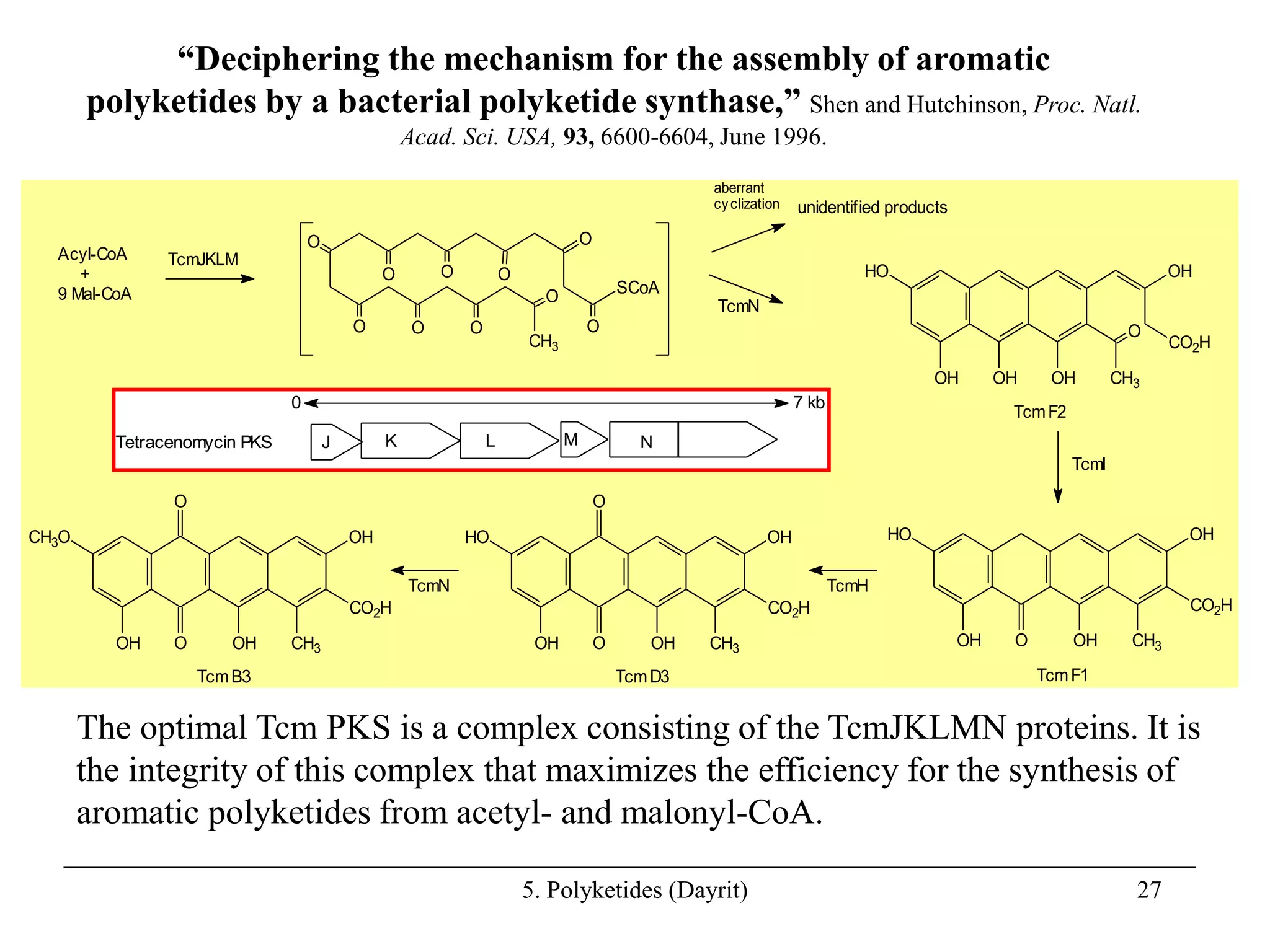
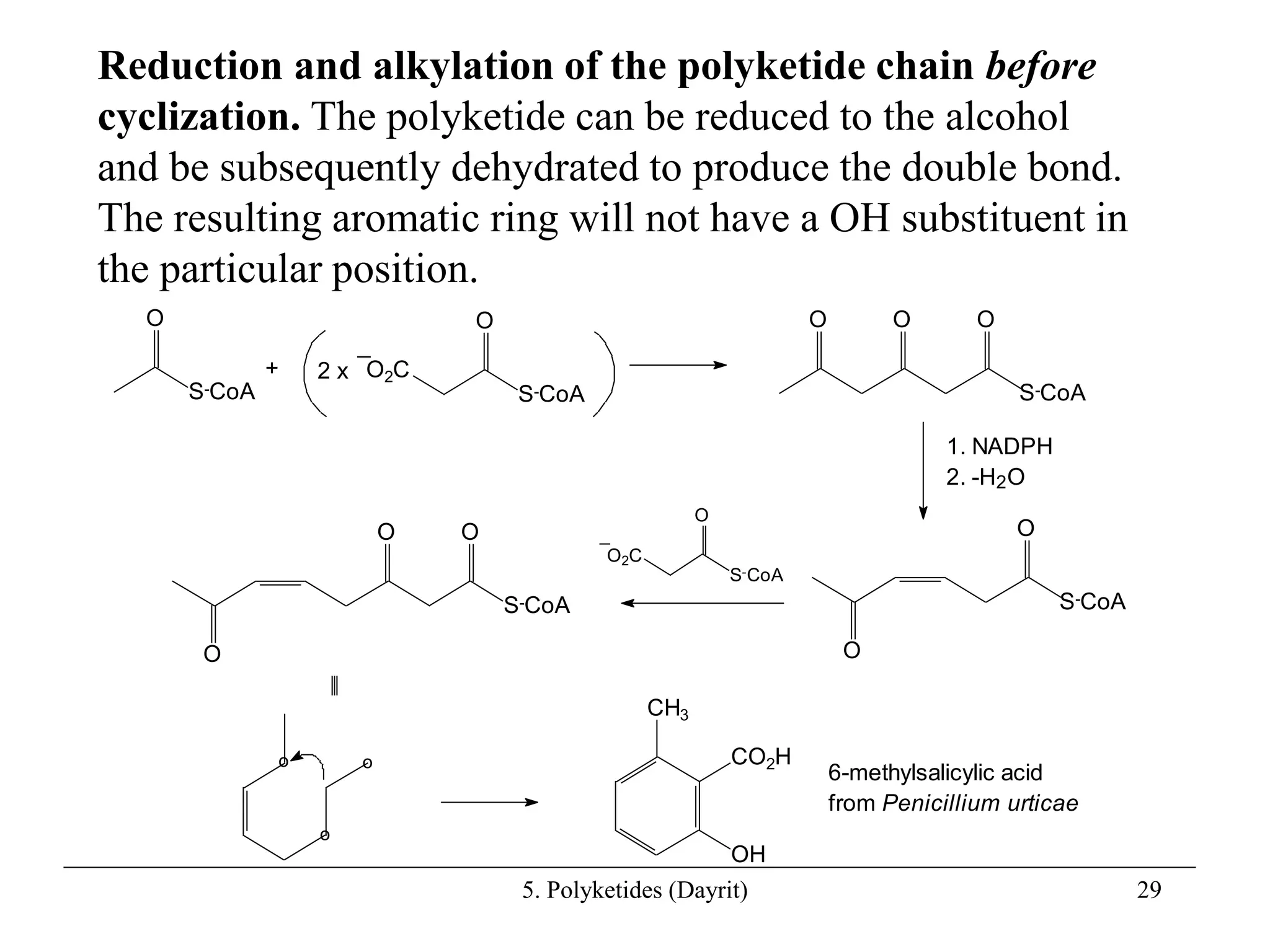
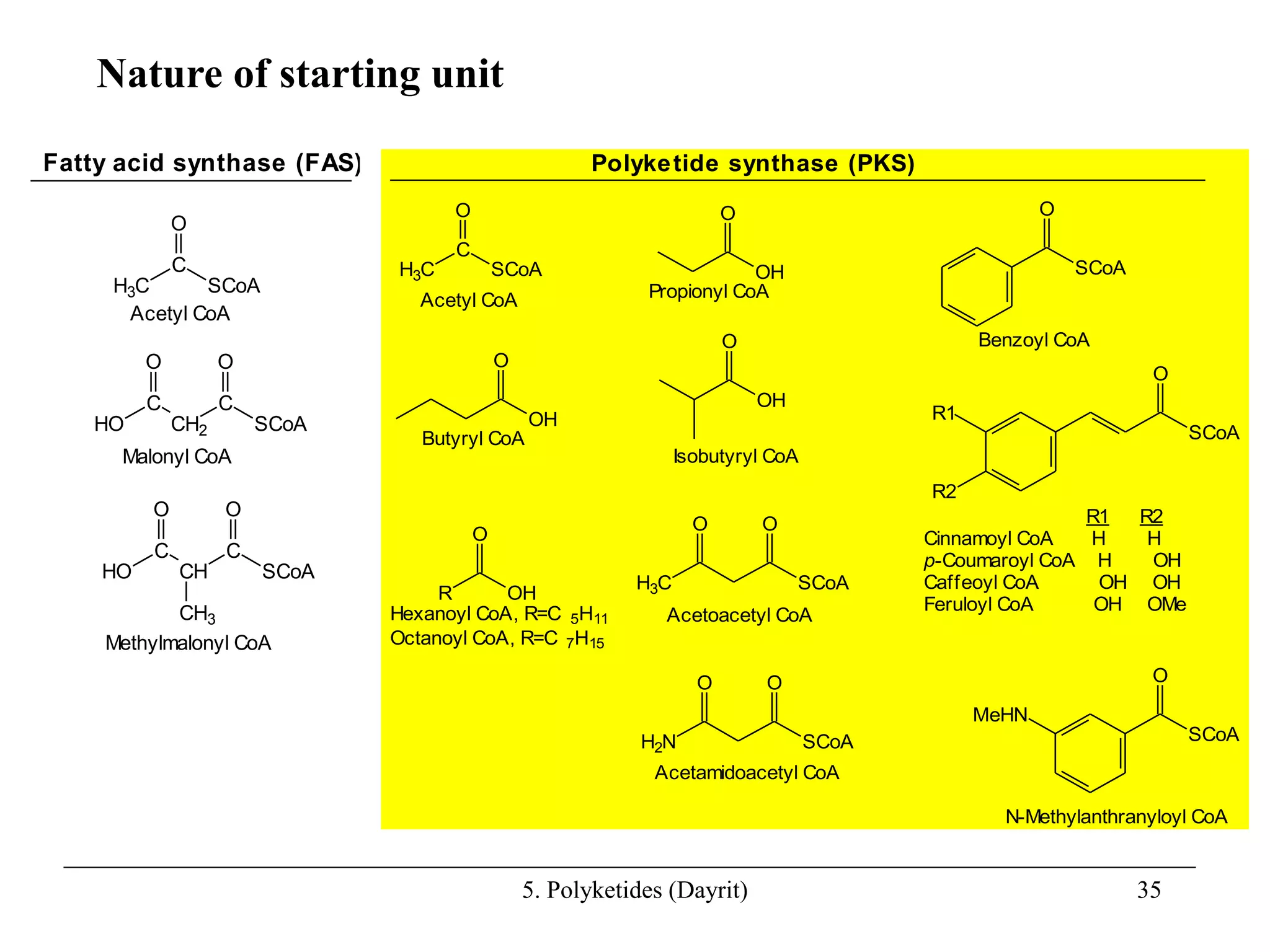
Polyketides are a diverse group of secondary metabolites produced through polyketide biosynthesis pathways that are similar to fatty acid synthesis. Polyketide pathways involve the polymerization of acetyl-CoA units to form linear polyketide chains that then undergo cyclization and other modifications to form a variety of aromatic and macrocyclic compounds with antibiotic and other bioactivities. These pathways are carried out by polyketide synthase enzyme complexes that assemble polyketide chains in an ordered fashion through the coordinated activities of ketosynthase, acyltransferase, and other functional domains.










![5. Polyketides (Dayrit) 13
Inter- vs.
intramolecular
cyclization:
A. Colletodiol;
B. Use of
labeling
experiments to
distinguish
intra- from
intermolecular
cyclization.
o o o
o o o o
O O
O
O
OH
OH
colletodiol
A. Example of intermolecular cyclization.
B. Use of labeling experiment to distinguish inter- vs. intramolecular cyclization.
o
o
o
o
o
o
o
o o o o
o
o
o
o
[Me*]
OH OH O
*
-CO2
2
-2CO](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-13-2048.jpg)












![5. Polyketides (Dayrit) 30
Reduction and alkylation of the polyketide chain before
cyclization. The polyketide can be C-alkylated (e.g., with
methyl or isopentyl groups) prior to cyclization although it
may be difficult to determine whether C-alkylation is carried
out before or after cyclization.
o
o
o o
[CH ]
3
3
[CH ]
CH3
OH
H3C
HO
CH3
O
clavatol
CH3
OH
HO
O
CH3
OH
H3C
HO
O](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-30-2048.jpg)
![5. Polyketides (Dayrit) 31
Secondary processes: examples of oxidation, decarboxylation and methylation.
6-methylsalicylic acid
CH3
CO2H
OH
[O]
CHO
CO2H
OH
-CO2
CHO
OH
CO2H
OH
HO
[O]
gentisic acid
A. Gentisic acid
B. Fumigatin
fumigatin
[CH ]
CH3
OH
OCH3
HO
HO
2
-CO
CH3
OH
HO
1. [O]
2.
CH3
CO2H
OH
HO
orsellinic acid
3 [O]
CH3
O
OCH3
HO
O](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-31-2048.jpg)


![5. Polyketides (Dayrit) 34
Intramolecular aromatic radical coupling: biosynthesis of griseofulvin (from a fungus, Penicillium
griseofulvum) involves extensive secondary modification of a heptaketide.
griseofulvin
O
OH
CH3O O
OCH3
O
CH3
Cl
+2 [H]
dehydrogriseofulvin
O
OH
CH3O O
OCH3
O
CH3
Cl
O
OH
CH3
O O
OCH3
O
CH3
Cl
.
.
.
.
O
OH
CH3
O O
OCH3
O
CH3
Cl
[O]
3
+[CH ]
griseophenone A
O
OCH3
CH3
O OH
OCH3
OH
CH3
Cl
+[Cl],
-[H]
O
OH
CH3
O OH
OCH3
OH
CH3
Cl
griseophenone B griseophenone C
O
OH
CH3O OH
OCH3
OH
CH3
3
+2 [CH ]
O
OH
HO OH
OH
OH
CH3
o o
o
o
o
o o](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-34-2048.jpg)


![39
Overview of biosynthesis
of quinones. Depending
on the organism,
quinones can arise via
the polyketide or
shikimate pathways.
In microorganisms:
[O]
polyketide aromatic compound quinone
In plants:
[O]
polyketide aromatic compound quinone
shikimate aromatic compound
+ terpene
[O]
quinone
(mixed metabolite)
quinone
OH
CO2H
OH
OH
OH
O
O
OH
H
quinones from shikimate + terpene:
quinone from shikimate:
homogentisic acid alkarinin
R
O
O
H
n
ubiquinones: R = H, CH ; n = 4-13
3
• Aromatic metabolites in
microorganisms are likely
to be formed via the
polyketide pathway while
aromatic compounds in
plants are likely to come
from the shikimate
pathway.](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-39-2048.jpg)





![5. Polyketides (Dayrit) 46
o
o
o
o o o o
o o o
decaketide
[O]
HO
OH
O
O OH
OH
O O O
+2[H] +2[H], -H O, +2[H]
2
HO
OH
O
O OH
OH
OH
O
H
+
HO
OH
O
O OH
O
OH
HO
HO
OH
O
O OH
O
O
-H O
2
averufin [O]
[O] HO
OH
O
O OH
OH
OH
O - H
OH
O
-H O
2
O - H
O
OH
OH
O
O
OH
HO
CHO
-C2
OH
OH
O
O
OH
HO
CHO CHO
OH
O
O
OH
HO O O
versicolorin A
[O], Bayer-Villiger
versicolorin B
OH
O
O
OH
HO O O
Aflatoxins
make up a
family of
polyketide
metabolites.
The very
complex
biosynthesis
of aflatoxins
was
elucidated
by George
Büchi.](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-46-2048.jpg)
![5. Polyketides (Dayrit) 47
versicolorin A
OH
O
O
OH
HO O O [O]
Bayer-Villiger
OH
O
OH
HO O O
CO2H HO
+2[H]
+2[H],
-CO2
OH
O
OH
HO O
O
OH
O
OH
O O
O
H
H
sterigmatocystin
OH
O
OH
O O
O
H
H
O
[O]
OH
O
OH
O O
O
H
H
O
[O]
[O]
OH
O
O
HO2C
O O
O
H
H
O
OH
O
O O
O
H
H
O
CO2H
O
_
OH
OH
O O
O
H
H
O
CO2H
O
[CH ]
3
-CO ,
+[CH ],
-H O
2
2
3
OCH3
O O
O
H
H
O
O
aflatoxin B1](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-47-2048.jpg)
![5. Polyketides (Dayrit) 48
o
o
o
o o o o
o o o
decaketide
[O]
HO
OH
O
O OH
OH
O O O
+2[H] +2[H], -H O, +2[H]
2
HO
OH
O
O OH
OH
OH
O
H
+
HO
OH
O
O OH
O
OH
HO
HO
OH
O
O OH
O
O
-H O
2
averufin [O]
[O] HO
OH
O
O OH
OH
OH
O - H
OH
O
-H O
2
O - H
O
OH
O
HO
-C2
OH
O
HO
CHO CHO](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-48-2048.jpg)
![5. Polyketides (Dayrit) 49
OH O OH OH
OH O OH OH
HO
OH
O
O OH
O
O
-H O
2
averufin [O]
[O] HO
OH
O
O OH
OH
OH
O - H
OH
O
-H O
2
O - H
O
OH
OH
O
O
OH
HO
CHO
-C2
OH
OH
O
O
OH
HO
CHO CHO
OH
O
O
OH
HO O O
versicolorin A
[O], Bayer-Villiger
versicolorin B
OH
O
O
OH
HO O O](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-49-2048.jpg)
![5. Polyketides (Dayrit) 50
versicolorin A
OH
O
O
OH
HO O O [O]
Bayer-Villiger
OH
O
OH
HO O O
CO2H HO
+2[H]
+2[H],
-CO2
OH
O
OH
HO O
O
OH
O
OH
O O
O
H
H
sterigmatocystin
OH
O
OH
O O
O
H
H
O
[O]
OH
O
OH
O O
O
H
H
O
[O]
[O]
OH
O
O
HO2C
O O
O
H
H
O](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-50-2048.jpg)
![5. Polyketides (Dayrit) 51
OH
O
OH
HO O
O
OH
O
OH
O O
O
H
H
sterigmatocystin
OH
O
OH
O O
O
H
H
O
[O]
OH
O
OH
O O
O
H
H
O
[O]
[O]
OH
O
O
HO2C
O O
O
H
H
O
OH
O
O O
O
H
H
O
CO2H
O
_
OH
OH
O O
O
H
H
O
CO2H
O
[CH ]
3
-CO ,
+[CH ],
-H O
2
2
3
OCH3
O O
O
H
H
O
O
aflatoxin B1](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-51-2048.jpg)
![5. Polyketides (Dayrit) 52
Biosynthesis of
tetracyclines from
Streptomyces
species.
R=H : tetracycline
R=OH : terramycin
OH O OH O
CONH2
OH
OH
H3C
OH
H
N(CH3)2
R
o
o
o
o
o
o o o o
+2[H] [CH ] [O]
CONH2
3
NH2
OH
HO
CH3
HO OH OH O
OH
[O]
NH2
OH
HO
CH3
HO OH O O
O
NH2
OH
HO
CH3
HO O O O
O
H H
NH2
OH
HO
CH3
HO O O O
O
H
OH
+H O
2
NH2
OH
HO
CH3
HO O O O
O
H
OH
+2[H]
NH2
OH
HO
CH3
HO O O O
H
OH
OH
+[NH ],
+2[CH ]
2
3
NH2
OH
HO
CH3
HO O O O
H
OH
N(CH3)2
A B C D
NH2
OH
HO
CH3
HO O O O
H
OH
N(CH3)2
Cl
D
C
B
A
Cl
OH O OH O
CONH2
OH
OH
H3C
OH
H
N(CH3)2
aureomycin
[Cl]](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-52-2048.jpg)
![5. Polyketides (Dayrit) 53
o
o
o
o
o
o o o o
+2[H] [CH ] [O]
CONH2
3
NH2
OH
HO
CH3
HO OH OH O
OH
[O]
NH2
OH
HO
CH3
HO OH O O
O
NH2
OH
HO
CH3
HO O O O
O
H H
NH2
OH
HO
CH3
HO O O O
O
H
OH
+H O
2
NH2
OH
HO
CH3
HO O O O
O
H
OH
+2[H]
NH2
OH
HO
CH3
HO O O O
H
OH
OH
+[NH ],
+2[CH ]
2
3
NH2
OH
HO
CH3
HO O O O
H
OH
N(CH3)2
OH
CH3
H
N(CH3)2
Cl
[Cl]
Biosynthesis of
tetracyclines
from
Streptomyces
species.](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-53-2048.jpg)
![5. Polyketides (Dayrit) 54
R=H : tetracycline
R=OH : terramycin
OH O OH O
CONH2
OH
OH
H3C
OH
H
N(CH3)2
R
HO HO OH O O
HO HO O O O
NH2
OH
HO
CH3
HO O O O
O
H
OH
+H O
2
NH2
OH
HO
CH3
HO O O O
O
H
OH
+2[H]
NH2
OH
HO
CH3
HO O O O
H
OH
OH
+[NH ],
+2[CH ]
2
3
NH2
OH
HO
CH3
HO O O O
H
OH
N(CH3)2
A B C D
NH2
OH
HO
CH3
HO O O O
H
OH
N(CH3)2
Cl
D
C
B
A
Cl
OH O OH O
CONH2
OH
OH
H3C
OH
H
N(CH3)2
aureomycin
[Cl]](https://image.slidesharecdn.com/050-polyketides-220720110349-d04e35e0/75/050-polyketides-ppt-54-2048.jpg)