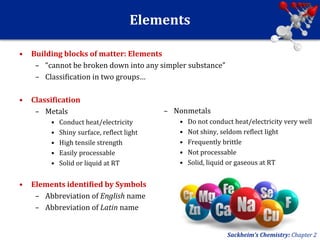
This document provides an overview of chemistry and physics concepts related to matter. It defines matter as anything that occupies space and has mass. Key topics covered include the states of solid, liquid, and gas; chemical and physical changes; elements, compounds, and mixtures; and the composition and properties of matter. Important chemistry concepts like the law of conservation of mass and the law of definite proportions are also summarized.